Plasma Lipoproteins
The lipoproteins in the circulation are complex aggregates of lipids and proteins that are not linked covalently to each other. They render the hydrophobic lipids compatible with the aqueous environment of body fluids and enable their transport throughout the body to tissues where they are required. Synthesis occurs mainly in the intestines and liver in all vertebrates and insects. Because of their clinical importance, a very high proportion of research on lipoproteins deals with their metabolism in humans in relation to health, and the discussion that follows reflects this. Within the circulation, these aggregates are in a state of constant flux, changing in composition and physical structure as the peripheral tissues take up the various components before the remnants return to the liver. The most abundant lipid constituents are triacylglycerols, free cholesterol, cholesterol esters and phospholipids (mainly phosphatidylcholine and sphingomyelin), although fat-soluble vitamins and antioxidants are also transported in this way (together with proteins/enzymes). Free (unesterified) fatty acids and lysophosphatidylcholine are bound to the protein albumin by hydrophobic forces in plasma and in effect are detoxified.
These lipoproteins are structurally and metabolically distinct from the proteolipids containing covalently linked fatty acids or other lipid moieties that are described on another web page.
1. Composition and Structure
Lipoprotein classes: Ideally, the lipoprotein assemblies should be described in terms of the main protein components known as apoproteins (or 'apolipoproteins'), as these determine the overall structures and metabolism and the interactions with receptor molecules in liver and peripheral tissues. However, the practical methods that have been used to separate different lipoprotein classes for study have determined the nomenclature. Thus, the main groups are classified as chylomicrons (CM), very-low-density lipoproteins (VLDL), low-density lipoproteins (LDL) and high-density lipoproteins (HDL), based on the relative densities of the aggregates on ultracentrifugation but conveniently with broadly separate functions. These classes can be further refined by improved separation procedures, and intermediate-density lipoproteins (IDL) and subdivisions of the HDL are often defined with characteristic apoprotein compositions and properties. Density is determined largely by the relative concentrations of triacylglycerols (lighter) and proteins and by the diameters of the broadly spherical particles, which vary from about 6000Å in CM to 100Å or less in the smallest HDL. Some compositional details are listed in Table 1.
Table 1. Physical properties and lipid compositions of lipoprotein classes. |
||||
| CM | VLDL | LDL | HDL | |
|---|---|---|---|---|
| Density (g/ml) | < 0.94 | 0.94-1.006 | 1.006-1.063 | 1.063-1.210 |
| Diameter (Å) | 6000-2000 | 600 | 250 | 70-120 |
| Total lipid (wt%) * | 99 | 91 | 80 | 44 |
| Triacylglycerols | 85 | 55 | 10 | 6 |
| Cholesterol esters | 3 | 18 | 50 | 40 |
| Cholesterol | 2 | 7 | 11 | 7 |
| Phospholipids | 8 | 20 | 29 | 46 |
| * Most of the remaining material comprises the various apoproteins. | ||||
The data for the relative compositions of the various lipid components should not be considered as absolute as they are in a state of constant flux, but in general the lower the density class, the higher the proportion of triacylglycerols and the lower the proportions of phospholipids and other lipid classes. In fact, the VLDL and LDL exhibit a continuum of decreasing size and density.
The fatty acid compositions of the main lipid classes in human lipoproteins are listed in Table 2. As might be expected, the triacylglycerols tend to contain a high proportion of saturated and monoenoic fatty acids, while the phospholipids have the highest proportion of polyunsaturated acids, especially arachidonate. Cholesterol esters are enriched in linoleate, reflecting their biosynthetic origin (see below). The composition of the triacylglycerols of the chylomicrons (not listed) depends largely on that of the dietary fatty acids. In general, minor differences only occur for the fatty acid compositions of each lipid between lipoprotein classes.
Table 2. Fatty acid compositions (wt% of the total) in the main lipids of human lipoprotein classes.* |
|||||||||
| Triacylglycerols | Cholesterol esters | Phospholipids | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VLDL | LDL | HDL | VLDL | LDL | HDL | VLDL | LDL | HDL | |
| 16:0 | 27 | 23 | 23 | 12 | 11 | 11 | 34 | 36 | 32 |
| 18:0 | 3 | 3 | 4 | 1 | 1 | 1 | 15 | 14 | 14 |
| 18:1 | 45 | 47 | 44 | 26 | 22 | 22 | 12 | 12 | 12 |
| 18:2 | 16 | 16 | 16 | 52 | 60 | 55 | 20 | 19 | 21 |
| 20:4(n-6) | 2 | 5 | 8 | 6 | 7 | 6 | 14 | 13 | 16 |
| * From Skipski, V.P. In: Blood Lipids and Lipoproteins. Quantitation, Composition and Metabolism. pp. 471-483 (ed. G.J. Nelson, Wiley-Interscience, New York) (1972). | |||||||||
In plasma, ~90% of oxylipins are present in esterified lipids transported by lipoproteins, with much of the C20 to C22 polyunsaturated oxylipins in the phospholipids in HDL.
Apoproteins: Although a wide variety of proteins of various kinds are transported in the form of lipoprotein complexes, apoproteins are the defining components that are essential for their formation and subsequent metabolism. In general, these consist of a single polypeptide chain often with relatively little tertiary structure, and they are required to solubilize the non-polar lipids in the circulation and to recognize receptors, which direct their metabolism and that of the associated lipids to particular tissues and purposes. The various types with their main (but not exclusive) lipoprotein associations, molecular weights and broad functions are listed in Table 3.
Table 3. The main properties of the apoproteins.* |
|||
| Apoprotein | Molecular weight |
Lipoprotein | Function |
|---|---|---|---|
| Apo A1 | 28,100 | HDL, CM | Main structural protein. Lecithin:cholesterol acyltransferase (LCAT) activation. |
| Apo A2 | 17,400 | HDL, CM | Enhances hepatic lipase activity |
| Apo A4 | 46,000 | HDL, CM | May increase triacylglycerol secretion |
| Apo A5 | 39,000 | HDL, VLDL, CM | Enhances triacylglycerol uptake |
| Apo B48 | 241,000 | CM | Derived from Apo B100 – lacks the LDL receptor |
| Apo B100 | 512,000 | IDL, LDL, VLDL | Binds to LDL receptor |
| Apo C1 | 7,600 | HDL, VLDL, CM | Activates LCAT |
| Apo C2 | 8,900 | HDL, VLDL, CM | Activates lipoprotein lipase |
| Apo C3 | 8,700 | HDL, VLDL, CM | Inhibits lipoprotein lipase and controls triacylglycerol turnover |
| Apo D | 33,000 | HDL | Associated with LCAT, progesterone binding |
| Apo E | 34,000 | HDL, VLDL, CM | At least 3 forms. Binds to LDL receptor |
| Apo(a) | 300,000-800,000 | Lp(a) | Linked by disulfide bond to apo B100 and similar to plasminogen |
| Apo H, J, L | Poorly defined functions | ||
| Apo M | HDL | Transports sphingosine-1-phosphate | |
| * Roman numerals are often used to designate apoproteins (e.g., Apo AI, AII, AIII, etc) as an alternative. CM = chylomicrons |
|||
Apoproteins are necessary for many different purposes other than lipid transport, some of which are listed above (and are discussed further below); some are ligands for receptors on cell surfaces and specify the tissues to which the lipid components are delivered, while others are cofactors for lipases or regulate lipid metabolism in the plasma in various ways.
Apo B100 and apo B48 are large and water-insoluble, and they are the only non-exchangeable apoproteins, which are assembled into triacylglycerol-rich lipoproteins with their lipid components in the intestines and liver and can be considered defining constituents of the various LDL classes. With 4536 amino acid residues, apo B100 is synthesised in the liver and is one of the largest monomeric proteins known; cholesterol esters are required for proper folding. Apo B48 represents the N-terminal 48% of apo B100, and in humans, it is produced only in the small intestine in response to fat in the diet. These apoproteins stay with their lipid aggregates during their passage in plasma and the various metabolic changes that occur, until they are removed eventually via their characteristic receptors.
The remaining soluble or exchangeable apoproteins, such as apo E, apo A4, apo C3, apo A5 and apo A1, are much smaller in molecular weight, and they can exchange between lipoprotein classes and acquire lipids during circulation.
Apo A1 is the main protein component of HDL and is synthesised within the liver (70%) and intestine (30%). It is a 28-kDa single polypeptide consisting of 243 amino acids, which has no disulfide linkages or glycosylation. Apart from the 44 amino acid N-terminal region, the protein is arranged as eight α-helical segments of 22 amino acids with two 11-mer repeats, and in some instances, these are separated by proline residues. It is believed that the helices are amphipathic, each with a hydrophobic face that interacts with lipids and a polar face that interacts with the aqueous phase. As the molecule probably exists in several conformational forms that can adapt to circumstances, it can stabilize all HDL subclasses. Some may enter the brain via the choroid plexus with the potential to influence brain lipid homeostasis. Aside from its role in lipoprotein metabolism, apo A1 modulates the inflammatory response by regulating the functions of immune cells, such as monocytes/macrophages, dendritic cells, neutrophils, and T lymphocytes, and it has an influence upon the metabolism of vascular endothelial cells and adipocytes.
Apo A2 is the second most abundant HDL apoprotein, and it exists as a homodimer with two polypeptide chains, each 77 amino acids in length and linked by a disulfide bond. Apo A4 is the largest member of the exchangeable apoprotein family and is a 376-amino acid glycoprotein, which is synthesised in intestinal enterocytes and secreted as a constituent of chylomicrons. In plasma, it transfers to HDL, but a high proportion is lipid free. Apo A5 is expressed in liver mainly, where it can associate with hepatic lipid droplets and has both extracellular and intracellular roles in triacylglycerol homeostasis. Although its concentration in plasma is relatively low, it is recognized as a potent regulator of plasma triacylglycerol levels through enhanced lipolysis and the clearance of lipoprotein remnants.
Apo C apoproteins are found in all lipoprotein classes. Apo C1 is the smallest exchangeable protein in this group and is synthesised in the liver, where like apo C3, the most abundant of the group, it inhibits lipoprotein lipase. Similarly, by inhibiting the binding of VLDL to the VLDL receptors, it increases plasma triacylglycerol levels, while by inhibiting the cholesterol ester transfer protein, it affects the metabolism of HDL. In contrast, apo C2 acts separately as an activator of lipoprotein lipase.
Apo D is atypical in that it is very different in structure from other apoproteins, and it is expressed widely in mammalian tissues (most others are produced mainly in liver and intestine). In plasma, it is present mainly in HDL and to a lesser extent in LDL, where it is a multi-ligand binding protein capable of transporting small hydrophobic molecules such as arachidonic acid, steroid hormones and cholesterol for metabolism or signalling.
Apo E is an O-linked glycoprotein in three isoforms and is synthesised by many tissues, including liver, brain, adipose tissue and arterial wall, but most is present in plasma lipoproteins derived from the liver. It takes part in many aspects of lipid and lipoprotein homeostasis, both for the triacylglycerol-rich lipoproteins and HDL, and it is believed to have some non-lipid transport related functions, for example on the immune response and inflammation. That produced in adipose tissue may have a metabolic role in obesity. As most lipoproteins cannot cross the blood-brain barrier, apo E is synthesised in the brain where it is the main apoprotein and is of particular relevance to cholesterol metabolism. Apo A, apo C and apo D are also present in brain, but not apo B.
Lipoprotein structures: Lipoproteins are spherical (VLDL, LDL, HDL) to discoidal (nascent HDL) in shape with a core of non-polar lipids, triacylglycerols and cholesterol esters, and a surface monolayer, ~20Å thick, consisting of apoproteins, phospholipids and non-esterified cholesterol, which serves to obscure the hydrophobic lipids and present a hydrophilic face to the aqueous phase as illustrated schematically for a triacylglycerol-rich chylomicron below.

The physical properties of apoproteins enable them to bind readily at the interface between water and phospholipids and especially to those phospholipids on the surface of the lipoproteins. The resulting outer shell of amphipathic lipids and proteins solubilizes the hydrophobic lipid core in the aqueous environment. Each apoprotein, other than apo B100, tends to have a helical shape with a hydrophobic domain on one side that binds to the lipid core and a hydrophilic face that orientates to the aqueous phase. The polar nature of the surface monolayer prevents the lipoprotein particles from aggregating to form larger units. As the lipid compositions of the lipoproteins change during circulation throughout the body, the apoproteins can adapt to the altering affinities at the surface by changing conformation. Some have very little tertiary structure so are flexible, while apo A1 has a mobile or hinge domain.
LDL particles average 22 nm in diameter with roughly 3000 lipid molecules in total, and they contain a hydrophobic core of approximately 170 triacylglycerol, 1600 cholesterol ester and 200 unesterified cholesterol molecules. The amphipathic surface monolayer has a single copy of apo B100 together with about 700 phospholipid and 400 free cholesterol molecules. Phosphatidylcholine, about 450 molecules, and sphingomyelin, about 185 molecules, are the main phospholipids, together with smaller numbers of lysophosphatidylcholine, phosphatidylethanolamine and other lipid molecules. The structure and physical functions of LDLs depend mainly on the core–lipid composition and the conformation of the apo B-100, which can interact with extracellular membranes such as blood vessel intima where the LDL lipids are susceptible to modification, e.g., by acetylation, enzymatic digestion and oxidation.
In contrast, HDL are highly heterogeneous in terms of their size, lipid and protein contents and their physical properties, and they can be separated by various means, including ultracentrifugation, immunoaffinity, precipitation and gel filtration, into subclasses with differences in composition. These have been designated in various ways, e.g., HDL1, HDL2, HDL3, etc., but there is no standard nomenclature or definition as the method of isolation affects the composition. As a useful clinical tool, they are probably best classified as very large HDL, large HDL, medium HDL, small HDL and very small HDL. Discoidal nascent HDL particles are believed to consist of a small unilamellar bilayer, containing approximately 160 molecules of phospholipid, which is surrounded by four apoprotein molecules, including at least two apo A1 monomers. Although most HDL particles in human plasma are spherical, the structures are poorly characterized in comparison to discoidal HDL, and it is believed that the apo A1 molecule changes conformation from the discoidal state and adopts a helical structure with the C‑terminal domain binding to the phospholipids. As well as apo A1 and other apoproteins (mainly apo C and E), HDL carry many other proteins/enzymes (~100) necessary for other purposes, and these are not evenly distributed across subfractions. They include lipases, acyltransferases and transport proteins, together with some having anti-oxidative or anti-inflammatory properties and some concerned with metabolic processes that do not concern lipids.
Lipoproteins are often categorized simplistically according to their main metabolic functions. The principal role of the chylomicrons and VLDL is to transport triacylglycerols ‘forward’ as a source of fatty acids from the intestines or liver to the peripheral tissues, and in contrast, the HDL remove excess cholesterol from peripheral tissues and deliver it to the liver where some is excreted in bile in the form of bile acids (‘reverse cholesterol transport’). While these are considered separately for ease of discussion in the account that follows, it should be recognized that the processes are highly complex and inter-related with transfer of apoproteins, enzymes and lipid constituents among the heterogeneous mix of all the lipoprotein fractions.
As birds, amphibians, fish and even round worms have lipoprotein systems comparable to those in mammals, it is evident that these must have developed early in evolution. The equivalent protein in these species is vitellogin, which is closely related to mammalian apo B.
Lipoprotein(a) (Lp(a)) differs structurally and metabolically from the other lipoproteins, and it consists of an LDL-like particle containing a highly polymorphic glycoprotein named apolipoprotein(a) (apo(a)), which is covalently bound via a disulfide bond to apo B100; it is found only in primates. While its physiological function is uncertain, Lp(a) is of particular interest because it has prothrombotic, proinflammatory and proatherogenic properties independently of other lipoproteins, in part because it can carry an appreciable load of oxidized phospholipids (see below). Biosynthesis of occurs almost entirely in the liver with synthesis of apo(a) in hepatocytes. Apo(a) should not be confused with apo A.
Cerebrospinal fluid: In humans, cerebrospinal fluid contains a heterogeneous range of lipoprotein subspecies enriched in proteins necessary for the health of the central nervous system, but at only 1% of the levels in plasma. They appear to resemble plasma HDL, although they are somewhat larger, and they are enriched in proteins for wound healing, inflammation, the immune response, and neuron generation and development.
2. Lipoprotein and Triacylglycerol Metabolism
Triacylglycerols are the most energy-dense molecules available to the body as a source of fuel but are highly hydrophobic. For efficient transport from the intestine and the liver to other organs of the body, they must be packaged in a form compatible with the aqueous environment in plasma, i.e., in lipoproteins, mainly chylomicrons and VLDL, although some proteins that are shared with HDL are necessary for the process to operate normally. Exchangeable apoproteins protect triacylglycerol-rich particles from non-specific interactions in plasma and ensure that they have the correct configuration to be acted upon by lipases.
Chylomicron formation: Dietary fatty acids and monoacylglycerols are absorbed by the enterocytes in the intestines, where they must cross the cytoplasm to the endoplasmic reticulum with the aid of fatty acid binding proteins. They are utilized immediately to form new triacylglycerols (see our web page on triacylglycerol biosynthesis), mainly by the monoacylglycerol pathway, and are thus detoxified. Together with dietary cholesterol, much of which is in cholesterol ester form, these triacylglycerols are incorporated into spherical chylomicron particles, which have a surface layer of phospholipids with a single molecule of the truncated form of apo B, apo B48, which is diagnostic for triacylglycerol-rich lipoproteins of intestinal origin. Chylomicrons are the largest lipoproteins present in the circulation, with their size dependent on the fed/fasted state, the rate of absorption of fat, and the type and amount of fat absorbed.
The synthesis of apo B100 and its truncated form and the accumulation of lipids to form chylomicrons or VLDL in intestinal cells and liver, respectively, are complex processes that are still only partly understood. Simplistically, secretory proteins such as apo B are synthesised on ribosomes on the surface of the endoplasmic reticulum and are translocated through the membrane to the lumen of the ER where VLDL are assembled by accretion of lipids with the aid of a microsomal triacylglycerol transfer protein (MTTP), which transfers phospholipids and triacylglycerols to nascent apo B for the assembly of lipoproteins. This occurs in three stages - pre-VLDL (pre-chylomicrons - nascent lipoproteins), VLDL2, a triacylglycerol-poor form of VLDL that is assembled in the Golgi and is transported to the basolateral membrane, where the final triacylglycerol-rich VLDL1 or chylomicrons with apo B48 and apo A4 are secreted by a process of reverse exocytosis into the intestinal lamina propria. Apo A1 is generated separately in the endoplasmic reticulum of enterocytes, and it is transported to the Golgi and added to the chylomicrons just before the mature particle is secreted into the lymph.
The chylomicrons are transported via the intestinal lymphatic system and enter the blood stream at the left subclavian vein. During circulation throughout the body, triacylglycerols are removed by peripheral tissues by the action of endothelial-bound lipoprotein lipase with entry of fatty acids into muscle for energy production and adipocytes for storage, while the apo B48 remains with the residual particle. The chylomicrons contain some apo A1, which is synthesised in the intestines and liver, but this is transferred spontaneously to the HDL as soon as the chylomicrons reach the circulation, while transfer of apo E and apo C(1-3) in the reverse direction from the HDL to the surface of the chylomicrons, displacing apo A4, occurs at the same time. Depleted or ‘remnant’ chylomicrons, containing the dietary cholesterol, apo E and apo B48 mainly, eventually reach the liver where they are cleared from the circulation by a receptor-mediated process that requires the presence of apo E.
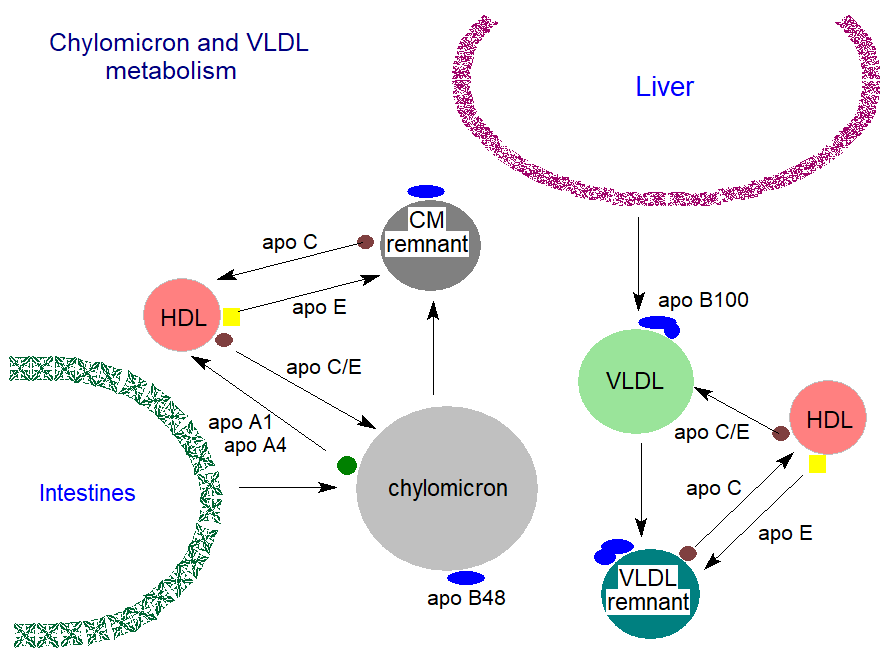
Liver catabolism: A high proportion of the VLDL remnants (or ‘IDL’) with apo B100 and apo E as the remaining proteins are sequestered in the liver perisinusoidal space (space of Disse), where they may undergo additional processing by lipases with a further loss of triacylglycerols as they are converted to LDL. Both apoproteins are required for recognition of the VLDL remnants and LDL by the LDL receptors in the liver, although many other tissues contain analogous receptors. The main LDL receptor in liver is a polypeptide of 839 amino acids, to which complex carbohydrate moieties are linked that spans the plasma membrane, and it has an extracellular domain responsible for binding to apo B100 and apo E. Within the cell, the receptors cluster into regions of the plasma membrane known as ‘coated pits’, where the cytoplasmic leaflet is coated with the protein clathrin. After binding of the LDL and some of the VLDL remnants to the receptor, the LDL-receptor complexes are internalized by endocytosis of the coated pit and then are dissociated by means of an ATP-dependent proton pump, which lowers the pH in the endosomes, enabling the receptors to be recycled to the plasma membrane. The LDL-containing endosomes fuse with lysosomes, and lipolytic enzymes such as a lysosomal acid lipase (LAL) release free fatty acids and cholesterol from triacylglycerols and cholesterol esters, while acid hydrolases degrade the apoproteins. Much of the apo E is believed to escape this process and is returned to the circulation and the HDL. A further receptor, the LDL-receptor-related-protein, assists in the removal of chylomicron remnants.
After their release from lysosomes, the fatty acids and other lipid components serve as precursors for the synthesis of new lipid species and may be needed for the regulation of many metabolic processes. For example, unesterified fatty acids are able to interact with the peroxisome proliferator-activated receptor PPARα and so target gene expression.
Secretion from the liver: The triacylglycerols of the remnant chylomicrons, together with cholesterol and cholesterol esters, are secreted by the liver into the circulation in the form of VLDL, which contain one molecule of the full-length form of apo B. Much of the triacylglycerols in VLDL are synthesised in the liver from free fatty acids reaching it via the plasma in the post-absorptive and fasted states from adipose tissue, where it is stored in triacylglycerol form in lipid droplets for mobilization upon demand. Liver lipid droplets and VLDL thereby serve to buffer the plasma free fatty acids released by lipolysis in adipose tissue in excess of the requirements of muscle and liver.
Within the liver, the nascent VLDLs are assembled from apo B100 and lipids, which consist largely of triacylglycerol droplets with some phospholipid, in the endoplasmic reticulum with the aid of a chaperone, namely microsomal triglyceride transfer protein (MTTP), before they are transported to the Golgi in a complex multistep process that utilizes a VLDL transport vesicle. In the lumen of the cis-Golgi, VLDLs undergo many modifications before they are transported to the plasma membrane and secreted into the circulatory system. The surface layer of the newly synthesised VLDL is enriched in phosphatidylethanolamine, which rapidly exchanges with phosphatidylcholine of other lipoproteins. The newly synthesised VLDL contain a little apo C3, apo E and apo A5, which may have a role in the assembly process, but they rapidly take up apo C2 (10-20 molecules) and apo E from HDL after a few minutes in the circulation, while the small amount of apo A1 of intestinal origin is transferred to HDL.
Lipoprotein lipase, the key enzyme in the peripheral tissues that is responsible for the hydrolysis of triacylglycerols from the chylomicrons and VLDL, is bound to the vascular surface of the endothelial cells of the capillaries of adipose tissue, heart and skeletal muscle, and lactating mammary gland primarily. It is a member of a lipase family that includes pancreatic lipase and hepatic triacylglycerol lipase and has an amino-terminal α/β-hydrolase domain harbouring a catalytic triad and a carboxyl-terminal β-barrel domain that interacts with its substrate. The enzyme is synthesised in the endoplasmic reticulum where it is activated by lipase maturation factor 1 (LMF1), before the complex is stabilized with other chaperones so that it attains the required tertiary fold for transport to the luminal surface of endothelial cells and into the interstitial space in the form of a monomer (not as a homodimer as was once believed). A small glycosylphosphatidylinositol-anchored protein designated GPIHBP1 binds the enzyme in an appropriate conformation in a 1:1 complex mediated via the carboxyl-terminal domain and facilitates the transfer of lipoprotein lipase across endothelial cells to the capillary lumen and then to the glycocalyx where it detaches. The enzyme is then in position to process the triacylglycerols of chylomicrons and LDL.
 Lipoprotein lipase
hydrolyses the primary ester linkages (positions sn-1/3) in triacylglycerols to produce free fatty acids and 2‑monoacylglycerols.
Then, the latter isomerize spontaneously to form 1/3-monoacylglycerols, which can likewise be hydrolysed by the enzyme.
However, monoacylglycerols can be taken up directly by cells and are not found in the remnant lipoproteins or bound to circulating albumin.
As the transport of VLDL particles progresses, the core of triacylglycerols is reduced and the proteins, including apo C2, and phospholipids
on the surface are transferred away to the HDL, although sufficient apo C2 remains to ensure that most of the triacylglycerols are removed.
As partially delipidated lipoproteins are detected in the circulation, it is believed that there is a process of dissociation and rebinding
to the enzyme, during each step of which triacylglycerols are hydrolysed and apo C2 is gradually released with formation of remnant particles.
Lipoprotein lipase takes part in the non-hydrolytic uptake of esters of cholesterol and retinol, possibly by facilitating transport.
Lipoprotein lipase
hydrolyses the primary ester linkages (positions sn-1/3) in triacylglycerols to produce free fatty acids and 2‑monoacylglycerols.
Then, the latter isomerize spontaneously to form 1/3-monoacylglycerols, which can likewise be hydrolysed by the enzyme.
However, monoacylglycerols can be taken up directly by cells and are not found in the remnant lipoproteins or bound to circulating albumin.
As the transport of VLDL particles progresses, the core of triacylglycerols is reduced and the proteins, including apo C2, and phospholipids
on the surface are transferred away to the HDL, although sufficient apo C2 remains to ensure that most of the triacylglycerols are removed.
As partially delipidated lipoproteins are detected in the circulation, it is believed that there is a process of dissociation and rebinding
to the enzyme, during each step of which triacylglycerols are hydrolysed and apo C2 is gradually released with formation of remnant particles.
Lipoprotein lipase takes part in the non-hydrolytic uptake of esters of cholesterol and retinol, possibly by facilitating transport.
Some of the unesterified fatty acids resulting from the action of lipoprotein lipase on VLDL triacylglycerols are taken up immediately by the cells by both receptor-mediated (CD36) and receptor-independent pathways, so they can be used for energy purposes or for the synthesis of other lipids, while the remainder is bound to circulating albumin from which it is released slowly to meet the cellular requirements of peripheral tissues. The glycerol produced is transported back to the liver and kidneys, where it can be converted to the glycolytic intermediate dihydroxyacetone phosphate. In muscle tissue, much of the fatty acids taken up are oxidized to two-carbon units for energy, but in adipose tissue triacylglycerols are formed for storage purposes, while in lactating mammary gland, they are used for milk fat synthesis. During fasting, hormone-sensitive lipase releases fatty acids from the triacylglycerols stores, and they are transported back into the circulation.
Apo C2 is an absolute requirement for activation of the enzyme, and there is evidence that this opens a lid-like region to enable the catalytic site to hydrolyse the fatty acid ester bonds of the triacylglycerols; apo A5 is also stimulatory. During lipolysis, several molecules of the enzyme, each with one molecule of apo C2, become attached to the surface of the chylomicron/VLDL particles simultaneously. Apo C1 and apo C3 inhibit lipoprotein lipase, in contrast, by competing for binding to lipoproteins rather than by deactivating the enzyme. Together with the inhibitory angiopoietin-like proteins ANGPTL3, ANGPTL4 and ANGPTL8, the apoproteins regulate lipoprotein lipase and thence triacylglycerol partitioning, hydrolysis and utilization. Apo C3 also inhibits the hepatic uptake of VLDL remnants and so has a controlling influence on the turnover of triacylglycerols. By slowing the clearance of triacylglycerols, high circulating levels of apo C3 are associated with elevated concentrations of blood lipids (hypertriglyceridemia) and are predictive of the risk of cardiovascular disease in humans. Improper regulation of lipoprotein lipase has been associated with the pathologies of atherosclerosis, coronary heart disease, cerebrovascular accidents, Alzheimer disease and chronic lymphocytic leukaemia.
It has been suggested that fatty acids released from VLDL triacylglycerols by lipoprotein lipase may act as signalling molecules to neurons and/or astrocytes in the brain, and these may in turn send information to peripheral tissues to maintain the energy balance at equilibrium.
3. VLDL, LDL and Cholesterol Metabolism
Cholesterol has a vital role in life and is essential for the normal functioning of cells both as a cell membrane constituent and as a precursor of steroid hormones and other vital metabolites. It can be supplied by the diet, but cholesterol biosynthesis de novo occurs in the liver, brain and at sites of major consumption, such as the adrenals, which use cholesterol as precursor for steroid biosynthesis. In most tissues, exchange to and from the lipoproteins occurs at the plasma membrane, but the blood-brain barrier prevents an exchange of cholesterol from the circulation. Much of the transfer occurs by receptor-mediated endocytosis and selective cholesteryl ester uptake from lipoprotein particles, but exchange of non-esterified cholesterol between lipoprotein particles and the cell membrane that is independent of receptors occurs also.
In the lumen of the small intestine, free cholesterol from the diet (animal-based foods) and from biliary secretion is solubilized in mixed micelles containing bile acids and phospholipids before it is absorbed by the enterocytes by a mechanism for which the apical protein Niemann-Pick C1-like 1 (NPC1L1) is crucial. Within the enterocyte, the metabolic fate of the absorbed cholesterol is determined by an integrated network of many different proteins. Most of it is transported to the endoplasmic reticulum where it is converted to cholesterol esters by the enzyme acyl-CoA:cholesterol acyltransferase 2 (ACAT2) and is selectively packaged into chylomicron particles, a process that requires a specific microsomal transfer protein and apoprotein B48, for transport out of the enterocyte into the lymphatic system and subsequently to the liver for uptake at the basolateral side of the hepatocytes, as described above for triacylglycerols. Regulation of intestinal cholesterol uptake and secretion is mediated by the nuclear receptor Liver X Receptor (LXR).
LDL are the main carriers of cholesterol from the liver to the adrenals and adipose tissue, where there are receptors requiring apo B100, and they can take in the LDL by a similar process to that occurring in liver. Within these tissues, the cholesterol esters are hydrolysed to yield free cholesterol, which is incorporated into the plasma membranes as required, while any excess cholesterol is re-esterified by an acyl-CoA:cholesterol acyltransferase for intracellular storage. Other peripheral tissues have much lower requirements for cholesterol, but that delivered by the LDL may be helpful in suppressing synthesis of cholesterol de novo within cells, and it may inhibit the expression of lipoprotein receptors.
The cholesterol at the particle surface enables VLDL to carry triacylglycerols efficiently in the aqueous environment of plasma. Once this has been accomplished, the resulting cholesterol-rich, triacylglycerol-depleted remnant LDL are potentially toxic and must be removed from the circulation. Some have argued that a significant part of the complexity of lipoprotein metabolism is concerned with the disposal of this LDL cholesterol before it can cause damage to the cardiovascular system. The liver is able to scavenge chylomicron remnants much more rapidly than LDL particles, and it seems likely that this specificity has evolved because the former are atherogenic. Therefore, further mechanisms such as that involving the HDL discussed next are required to return the excess LDL cholesterol to liver.
In macrophages, scavenger receptors mediate the uptake of LDL damaged by oxidation or other means to the point that its affinity to the LDL receptor is reduced with the unfortunate consequence that cholesterol can accumulate in macrophages in an unregulated manner, a possible first step in the development of atherosclerosis.
4. High-Density Lipoprotein and Cholesterol Metabolism
Measurement of HDL-associated cholesterol is often used as a standard method for assessing cardiovascular risk, and it may be relevant to non-vascular diseases, such as age-related macular disease, although many other factors are certainly involved. Mature HDL are the most complicated and diverse of the lipoproteins, as they contain many different protein constituents whose main purpose is to enable secretion of cholesterol from cells, esterification of cholesterol in plasma, transfer of cholesterol to other lipoproteins, and the return of cholesterol from peripheral tissues to the liver, and they undergo constant dynamic remodelling over their 4 to 5-day life cycle. This process that has been termed ‘reverse cholesterol transport’, as it may eventually lead to elimination of the excess of this lipid. In relation to triacylglycerol metabolism, HDL facilitate the activation of lipoprotein lipase, transfer triacylglycerols between lipoprotein classes, and assist in the removal of chylomicron remnants and VLDL enriched in triacylglycerols.
As well as the apoproteins, other components of HDL include anti-oxidative enzymes and phospholipid transfer proteins (PLTP). The latter mediate a net transfer of phospholipids from apo B-containing triacylglycerol-rich lipoproteins into HDL, and this is believed to be a factor in the enlargement of HDL. Apoproteins apo C1, apo C2, apo E and especially apo A2 also accumulate in HDL. As many of the lipid and protein constituents of HDL are exchangeable with other lipoproteins, many different types (subclasses) of HDL particle are generated with differing metabolic roles.
Nascent HDL are synthesised in the extracellular space of the liver and small intestine as protein-rich disc-shaped particles, but their compositions change and evolve as the HDL circulate in the plasma. Apo A1 synthesised in the liver together with that released spontaneously from chylomicrons is a key molecule that binds to phospholipids with a little cholesterol of cellular origin, and it has been described as the scaffold for HDL assembly and is secreted as pro-apo A1, which is rapidly cleaved by a circulating metalloproteinase to generate the mature polypeptide. It is believed that the origin of the cholesterol lies in caveolae (a type of membrane 'raft') in the plasma membrane, and the process is receptor-mediated via a transporter molecule ‘ABCA1’, one of a superfamily of 48 ABC membrane transporters that facilitates the transfer of phospholipids and cholesterol to lipid-poor apoproteins such as apo A1 in the nascent HDL (preβ-1 HDL) particles. A related but intracellular transporter ABCG1 stimulates the net outflow of cholesterol into larger HDL and helps to transfer cholesterol from the endoplasmic reticulum to the plasma membrane.
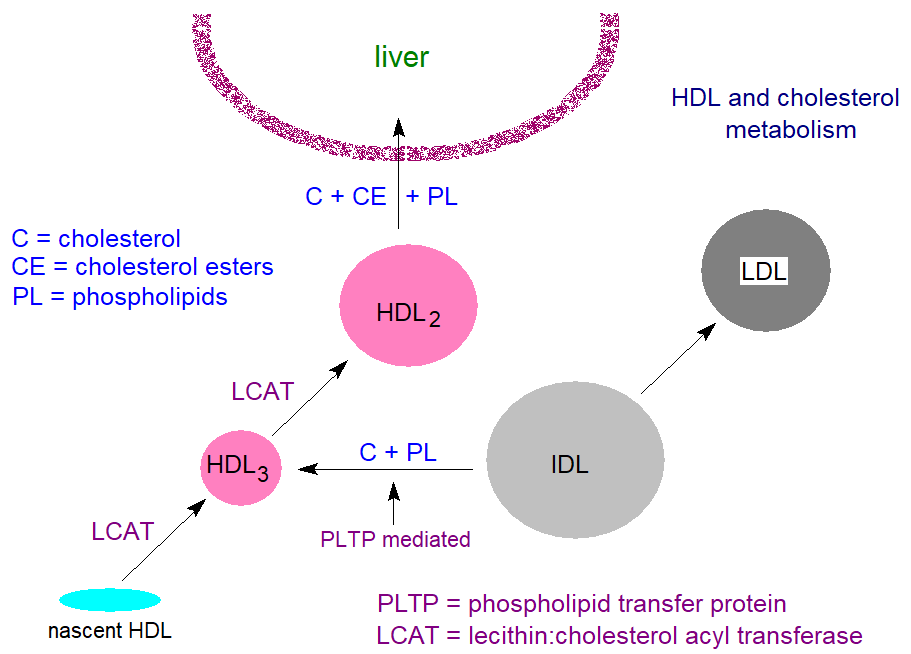
The further development of mature HDL is dependent on the enzyme lecithin:cholesterol acyltransferase (LCAT), which requires apo A1 for activation and is present mainly in the plasma compartment of the circulation (lecithin is an early trivial name for phosphatidylcholine). Our web page on cholesterol esters contains a description of the mechanism of action of this enzyme, but in brief it transfers a fatty acid from position sn-2 of phosphatidylcholine to the hydroxyl group of cholesterol, resulting in the formation of cholesterol esters and lysophosphatidylcholine. The cholesterol esters are highly hydrophobic and accumulate in the core of the HDL, while the lysophosphatidylcholine is removed from the HDL (and eventually from the plasma) by binding to albumin. By this means, LCAT enables the nascent HDL to continue to draw free cholesterol and phospholipids from IDL and LDL, the latter mediated by a phospholipid transfer protein, until spherical HDL particles are formed, i.e., HDL3 and HDL2, with a surface coat of phospholipid, free cholesterol and apoproteins.
Early in the formation of HDL, apo A2 is secreted from the liver and added to the surface, and those HDL particles enriched with apo A2 can stimulate various enzymes, including platelet-activating factor acetylhydrolase and lipoprotein-associated phospholipase A2, as well as exerting antioxidant effects. As the HDL develop, they acquire apo A4 and apo A5, the three apo C proteins and apo E from the VLDL and chylomicrons.
Cholesterol is obtained by extraction from surface membranes of cells of peripheral tissues by two basic mechanisms, although the relative contributions of each are uncertain. In energy dependent processes, the ABCA1 transporter carries cholesterol and phospholipid molecules to lipid-free apo A1 molecules, while the ABCG1 transporter transfers cholesterol to discoidal and spherical HDL molecules. A hydrophobic tunnel in the scavenger receptor BI molecule enables selective uptake and facilitated diffusion of cholesterol and cholesterol esters and does not require energy consumption. As a consequence, the levels of intracellular cholesterol are reduced as cholesterol stored in cells in the form of cholesterol esters is mobilized to replace that removed from the plasma membrane. It has long been believed that removal of excess cholesterol from macrophage-derived foam cells in atherosclerotic plaques and from arterial walls will result in atheroprotection, and conversely, that delayed cholesterol efflux from the arterial wall might impair reverse cholesterol transport and accelerate atherosclerosis. However, the failure of agents that raise HDL levels to reduce cardiovascular disease suggest the mechanism for cardioprotection may be more complex than this simplistic hypothesis.
 The liver is the major organ responsible for HDL clearance, and the entire HDL particle
can enter the hepatocytes through an apo A1-receptor interaction, where it undergoes a facilitated transfer of cholesterol
and cholesterol esters to distinct pools within the cell.
The modified HDL are secreted back into the circulation where they can acquire further cholesterol before returning to the liver.
In a second less-efficient pathway, the apo E in the HDL aids their uptake and catabolism by a process of
endocytosis via a receptor in a similar manner to that described above for LDL, which results in the degradation of all the HDL constituents.
This completes the process of reverse cholesterol transport.
Some of this cholesterol is converted to bile acids and exported into the intestines to aid digestion.
As a proportion of these are eventually excreted, it is a means of reducing total amount of cholesterol in the body.
The apo A1 recycles extracellularly between lipid-poor (pre-beta) and lipid-rich (spheroidal) lipoproteins, though de-lipidated apo
A1-particles are cleared by the kidneys preferentially.
The liver is the major organ responsible for HDL clearance, and the entire HDL particle
can enter the hepatocytes through an apo A1-receptor interaction, where it undergoes a facilitated transfer of cholesterol
and cholesterol esters to distinct pools within the cell.
The modified HDL are secreted back into the circulation where they can acquire further cholesterol before returning to the liver.
In a second less-efficient pathway, the apo E in the HDL aids their uptake and catabolism by a process of
endocytosis via a receptor in a similar manner to that described above for LDL, which results in the degradation of all the HDL constituents.
This completes the process of reverse cholesterol transport.
Some of this cholesterol is converted to bile acids and exported into the intestines to aid digestion.
As a proportion of these are eventually excreted, it is a means of reducing total amount of cholesterol in the body.
The apo A1 recycles extracellularly between lipid-poor (pre-beta) and lipid-rich (spheroidal) lipoproteins, though de-lipidated apo
A1-particles are cleared by the kidneys preferentially.
In many animal species, there is an alternative process in which cholesterol is obtained from plasma HDL by a mechanism whereby the lipoproteins bind to the surface of cells and part with their cholesteryl esters by a process known as the selective cholesterol uptake pathway, without the uptake and lysosomal degradation of the particle itself. This is a high capacity and highly regulated bulk delivery system for cholesterol that operates primarily but not exclusively in steroidogenic tissues to selectively internalize cholesterol esters and thence their cholesterol to produce steroid hormones, as in adrenal, ovarian and testicular tissues. In this pathway, scavenger receptor B type 1 (SR-B1) is the cell surface receptor responsible for selective uptake of HDL cholesterol esters. Unesterified cholesterol may be taken up from HDL by a related process in the liver for bile acid production. As part of this process, phosphatidylcholine in HDL is acquired by the liver, and in mice it has been demonstrated that half of the hepatic phosphatidylcholine is derived from the circulation; perhaps surprisingly, a high proportion of this is converted to triacylglycerols.
Cholesterol esters of HDL can be transferred to VLDL and LDL by the action of a bi-directional enzyme associated with HDL, the cholesterol ester transfer protein (CETP), and they can be exchanged for triacylglycerols by CETP from VLDL to HDL and vice versa. This means that excess cellular cholesterol can be returned to the liver by the LDL-receptor pathway as well as by the HDL-receptor pathway. The result is a net mass transfer of cholesterol esters from HDLs to VLDLs and LDLs and of triacylglycerols from VLDLs to LDLs and HDLs (inhibition of CEPT may have therapeutic potential towards cardiovascular and other diseases). Once in the HDL, triacylglycerols are rapidly broken down by various lipolytic enzymes, though the physiological significance of this in health terms at least is a matter of debate. There is a phospholipid transfer protein that mediates a net transfer of phospholipids from apo B-containing, triacylglycerol-rich lipoproteins into HDL and exchanges phospholipids between lipoproteins; it appears to be essential for maintaining normal HDL levels in plasma.
There are reports from experiments with animals and humans that some cholesterol is secreted directly into the intestines by a process known as trans-intestinal cholesterol efflux, but much of this may be re-absorbed under normal physiological conditions.
Following assembly, HDL particles undergo continual remodelling both lipids and proteins, through interactions with enzymes such as lipases, acyltransferases, lipid transfer proteins and scavenger receptors for proteins. Rearrangements probably occur within HDL particles through protein-protein, lipid-protein and lipid-lipid interactions. In addition to the main lipid and protein components, HDL transport small RNAs, hormones, carotenoids, vitamins and lipid mediators. As they can interact with most cells and deliver lipid-soluble cargo, HDL have the capacity to affect innumerable processes other than those concerned with cholesterol metabolism.
While the importance of HDL in the metabolism of cholesterol is undeniable, it may now be too simplistic an approach to consider only total HDL cholesterol as of clinical relevance, and there are suggestions that in consequence too little weight has been given to other functions of HDL. The role of HDL in acting as a circulating store of apo C1, apo C2 and apo E is highly relevant, although 60% of the proteins in HDL (200 identified to date) are not concerned with transport, and many are needed for other purposes, such as their anti-oxidative, anti-inflammatory, anti-apoptotic, anti-thrombotic, anti-infective and vasoprotective properties. Rather, individual subclasses of HDL with their own lipid and protein complements may need to be considered separately. For example, lipoproteins from the protein-rich HDL3 sub-fraction have a protective role against cardiovascular disease by acting as anti-inflammatory regulators to inhibit pro-inflammatory cytokines. Other HDL subclasses carry an enzyme that hydrolyses platelet activating factor (PAF-acetyl hydrolase), which is a potent phospholipid mediator that is pro-inflammatory.
HDL prevent the oxidation of LDL and limit the concentrations of oxidized components, which might otherwise render them atherogenic. Thus, human serum paraoxonase is a calcium-dependent enzyme associated with HDL, which catalyses the hydrolysis of oxidized fatty acids from phospholipids and prevents the accumulation of oxidized lipids in lipoproteins, especially LDL. On the other hand, while apo A1 is normally anti-inflammatory, it can be oxidized and then become pro-inflammatory with a reduced capacity for reverse cholesterol transport.
Brain: Lipoproteins are not able to cross the blood-brain barrier, but various brain cells are able to express lipoprotein receptors, lipid transporters and apoproteins, which are required for cholesterol turnover and biogenesis of lipoproteins. As the brain does not use triacylglycerols as an energy source, the lipid transport system is reliant on HDL or more accurately "HDL-like particles”, the concentration of which are much lower than the plasma equivalents. Brain HDL particles are heterogeneous in terms of density, size and composition, but most are spherical (13-20 nm) with a few that are disc-like. They are distinctive in that the main protein component is apo E, which is expressed and secreted from astrocytes, microglia and pericytes; some HDL containing apo A1 are spherical and smaller (10-18 nm). In brain, HDL have a vital role in supplying neurons with cholesterol, which is internalized by a receptor-mediated process as a crucial metabolite. They undergo biogenesis, maturation and remodelling processes that resemble those observed for plasma HDL, although they have not been as extensively studied. Cholesterol in brain undergoes unique oxygenation reactions, and the products are exported from brain across the blood-brain barrier to influence metabolism in other tissues (see our web page on oxysterols). Disturbances to brain HDL metabolism and cholesterol homeostasis may be a factor in neurodegenerative disorders such as Alzheimer's disease.
5. Lipoproteins and Disease
While only a few persons carry inherited defects in lipoprotein metabolism, such as hyper- or hypolipoproteinemias, abnormal lipoprotein metabolism is often observed as a secondary consequence of diabetes, hypothyroidism and kidney disease. In Tangier disease, patients have mutations in both copies of the genes that code for the ABCA1 transporter protein (see previous section), so they have very little circulating HDL. Similarly, defects in any of the enzymes for triacylglycerol transport and metabolism can lead to hypertriglyceridaemia with a severe impact upon health.
Oxidative stress can damage lipids carried by lipoproteins and indeed the protein components in plasma or when retained in the artery wall, leading to conversion of LDL into oxidized LDL (oxLDL), increased levels of which are strongly correlated to various diseases, including atherosclerosis, cancer and non-alcoholic steatohepatitis. Both HDL and LDL can carry phospholipids and other lipids in which the polyunsaturated fatty acid components are oxidized in various ways, and some of these oxidation products carry reactive (electronegative) aldehyde moieties that can form adducts with the amino acid residues of apolipoproteins (see our web page on oxidized phospholipids).
Under conditions of oxidative stress, the peptide chains of apoproteins, especially apo B, can also be subjected to oxidative modifications with consequences for plaque formation. All these reactions occur mainly within the subendothelial space of the arterial wall, although other deleterious reactions, such as glycation or homocysteinylation, occur in plasma. Circulating oxLDL may signal to cells at more distant sites and possibly trigger a systemic antioxidant defence, and they are recognized as biomarkers of atherosclerosis and metabolic disorders such as diabetes and obesity. Cross-linking of HDL apoproteins (apo-A1) by oxidized phospholipids, especially those that are oxidatively truncated and contain alkenyl aldehyde moieties, can occur while HDL are the major carrier of F2-isoprostanes in plasma. This can contribute to the loss of the atheroprotection by HDL in vivo, and indeed in some pathological conditions oxidized HDL can be proinflammatory.
Under normal physiological conditions, there is a response by phagocytes of the reticulo-endothelial system to remove oxLDL from circulation, assisted by Kupffer cells of the liver, sinusoidal endothelial cells and macrophages. However, when oxidatively stressed, these cells can be overwhelmed by oxLDL such that the excessive accumulation of lipids transforms them into foam cells, characteristic of the progression of atherosclerosis. The result is to reduce the affinity of oxLDL for LDL receptors, while increasing their recognition by scavenger receptors. For example, the lectin-like oxidized LDL receptor-1 (LOX-1) is a scavenger receptor that promotes endothelial dysfunction by inducing pro-atherogenic signalling and plaque formation through the endothelial uptake of oxLDL. This contributes to the initiation, progression and destabilization of atheromatous plaques and leads eventually to the development of myocardial infarction and certain forms of stroke. As LOX-1 is expressed in macrophages, cardiomyocytes, neutrophils and many other cell types, this receptor is further implicated in numerous aspects of atherosclerotic plaque formation.
The role of lipoproteins in the metabolism of triacylglycerol and cholesterol in relation to cardiovascular disease is highly complex and contentious. The problem is manifested first when LDL and other apo B-containing particles become oxidized, aggregated or enzymatically modified within the arterial wall and are no longer recognized by the LDL receptor. Instead, they are taken up by scavenger receptors expressed on macrophages and smooth muscle cells in the vascular wall, a process that is not regulated by a feedback mechanism. Cholesterol esters are hydrolysed by the lysosomal acid lipase and a massive accumulation of cholesterol can occur in these cells. The result is an increased transformation of macrophages into foam cells, which are the basis of atherosclerotic plaques, and an increased risk of coronary artery disease. Cholesterol efflux from cholesterol-loaded macrophage foam cells and other atherosclerosis-relevant cells requires an integrated web of interactions between apo A1, apo E, lecithin:cholesterol acyltransferase (LCAT), ATP binding cassette transporter A1 (ABCA1), scavenger receptor-B1 (SR-B1), and free cholesterol. Clinical manipulation of such complex processes to attenuate atherosclerosis is not straightforward.
 While
further discussion is best left to clinical specialists, suffice it to say that many epidemiological studies
have demonstrated that low concentrations of HDL cholesterol are associated with a higher risk of atherosclerosis;
conversely, high concentrations of HDL cholesterol are associated with a lower risk.
This is the so-called ‘good cholesterol’ of common parlance, which is being transported back to the liver for catabolism.
It is believed that HDL may protect against atherosclerosis via the promotion of reverse cholesterol transport.
In contrast, higher concentrations of LDL cholesterol have been associated with increasing severity of cardiovascular disease,
although the experimental correlations are not as good as for HDL; this is the ‘bad cholesterol’.
Unfortunately, drug treatments that have focused on raising HDL-cholesterol levels have failed to reduce morbidity and mortality
in coronary heart disease, and it seems probable that concentration on this factor alone may not be a suitable strategy
for the prevention and treatment of this condition.
Indeed, some consider that the triacylglycerol concentration of HDL may be a better practical marker for metabolic and cardiovascular risk.
While
further discussion is best left to clinical specialists, suffice it to say that many epidemiological studies
have demonstrated that low concentrations of HDL cholesterol are associated with a higher risk of atherosclerosis;
conversely, high concentrations of HDL cholesterol are associated with a lower risk.
This is the so-called ‘good cholesterol’ of common parlance, which is being transported back to the liver for catabolism.
It is believed that HDL may protect against atherosclerosis via the promotion of reverse cholesterol transport.
In contrast, higher concentrations of LDL cholesterol have been associated with increasing severity of cardiovascular disease,
although the experimental correlations are not as good as for HDL; this is the ‘bad cholesterol’.
Unfortunately, drug treatments that have focused on raising HDL-cholesterol levels have failed to reduce morbidity and mortality
in coronary heart disease, and it seems probable that concentration on this factor alone may not be a suitable strategy
for the prevention and treatment of this condition.
Indeed, some consider that the triacylglycerol concentration of HDL may be a better practical marker for metabolic and cardiovascular risk.
The apoproteins and associated enzymes of HDL are believed to be required for the maintenance of health in many further ways, including antioxidant, anti-inflammatory and anti-thrombotic effects, and it has been argued that the levels of apo B and of apo C3 in plasma may be good predictors of the risk of coronary heart disease. Apo B may mediate the interaction between LDL and the arterial wall so could initiate the development of atherosclerosis, while there is a clear link between apo C3 and the progression of cardiovascular disease and calcification of the aortic valve. Indeed, virtually all the apo B-containing lipoproteins can pass through the endothelial layer of arteries and initiate atherogenesis, but the smaller LDL are highly atherogenic because they enter the plaques with relative ease and have a high content of cholesterol. Thus, they provide substrates that trigger plaque initiation and growth.
Apo A4 has anti-oxidative and anti-inflammatory properties, and by its role in reverse-cholesterol transport, it may be protective against cardiovascular disease. It affects metabolism in relation to food intake, obesity and diabetes by acting as an acute satiation factor and by modulating glucose homeostasis.
In the brain, Apo E is important for cholesterol metabolism, but excess of the apo E4 form is associated with late onset Alzheimer's disease, where it is believed to influence the accumulation of the extracellular amyloid plaques composed of amyloid beta peptides that are the hallmark of the disease. This variant is a factor in other neurodegenerative diseases, and dysregulation of its expression can lead to accumulation of cytoplasmic lipid droplets, defects in endolysosomal trafficking and impaired mitochondrial metabolism in astrocytes and microglia. While apo E does not appear to affect overall levels of cholesterol and phospholipids in the central nervous system, it may modulate cholesterol and phospholipid homeostasis in particular subcellular membrane compartments. As apo E mediates sulfatide trafficking and metabolism in the brain, it is seen as a target for therapeutic intervention in diseases of the central nervous system. It is believed to influence susceptibility to parasitic, bacterial and viral infections, and in HIV-positive patients, apo E4 may hasten the progression to AIDS.
Lipoproteins and HDL especially have a role in host defence as part of the innate immune system. Infection and inflammation induce the acute-phase response, which leads to many changes in lipid and lipoprotein metabolism and initially protects the host from the harmful effects of bacteria, viruses and parasites, provided that the infections are not prolonged. For example, HDL and other lipoproteins bind the endotoxin lipopolysaccharides, which are primary constituents of the outer surface membrane of Gram-negative bacteria, and so neutralize their toxicity.
Lipoprotein(a): High concentrations of lipoprotein(a) (Lp(a)) in plasma have long been recognized to be a risk factor for cardiovascular disorders such as coronary heart disease and the development of aortic stenosis. One possible explanation for the atherogenicity of Lp(a) is that it is highly susceptible to oxidation and carries proinflammatory oxidized phospholipids, which are bound covalently to the apo(a) component. While the homology between apolipoprotein(a) and plasminogen suggests another link between the processes of atherosclerosis and thrombosis, it appears that it has yet to be demonstrated that this similarity has pathophysiological relevance in humans. It is evident that much of the metabolic fate of Lp(a) remains uncertain, and studies are hampered by the lack of a model experimental animal; it is almost certainly synthesised at the surface of the liver, or in an extra-hepatic space separated from the plasma, but how it is cleared from the circulation does not appear to be known.
6. Insect Lipoproteins
 Insects have a relatively simple lipoprotein
metabolism that serves as a useful model system for comparative studies (e.g., the vinegar fly Drosophila melanogaster).
The haemolymph, the circulatory fluid in insects, contains a single multifunctional lipoprotein termed lipophorin
that transports lipids to wherever they are required for energy and other purposes.
Rather than triacylglycerols, the main lipid components are 1,2-diacyl-sn-glycerols,
together with hydrocarbons, sterols and phospholipids, with phosphatidylethanolamine rather than phosphatidylcholine as the main phospholipid
class.
High-density lipophorin (HDLp) is the main lipoprotein class and it is composed of two integral and non-exchangeable apoproteins,
apolipophorin I (240 kDa) and apolipophorin II (80 kDa), which are apolipoprotein B homologues and are produced from a common precursor
by proteolytic cleavage.
Approximately 50% of the lipoprotein mass is comprised of lipids.
In contrast, the exchangeable apolipophorin-III is a lipid-free haemolymph protein that associates with lipophorin
during hormone-induced lipid mobilization and has functions beyond lipid transport.
Insects have a relatively simple lipoprotein
metabolism that serves as a useful model system for comparative studies (e.g., the vinegar fly Drosophila melanogaster).
The haemolymph, the circulatory fluid in insects, contains a single multifunctional lipoprotein termed lipophorin
that transports lipids to wherever they are required for energy and other purposes.
Rather than triacylglycerols, the main lipid components are 1,2-diacyl-sn-glycerols,
together with hydrocarbons, sterols and phospholipids, with phosphatidylethanolamine rather than phosphatidylcholine as the main phospholipid
class.
High-density lipophorin (HDLp) is the main lipoprotein class and it is composed of two integral and non-exchangeable apoproteins,
apolipophorin I (240 kDa) and apolipophorin II (80 kDa), which are apolipoprotein B homologues and are produced from a common precursor
by proteolytic cleavage.
Approximately 50% of the lipoprotein mass is comprised of lipids.
In contrast, the exchangeable apolipophorin-III is a lipid-free haemolymph protein that associates with lipophorin
during hormone-induced lipid mobilization and has functions beyond lipid transport.
Lipophorin transports dietary lipids from the insect gut to the fat body, the main metabolic organ that simplistically can be considered to combine the roles of the mammalian liver and adipose tissue, and thence to the peripheral tissues. Rather than being immediately internalized and the constituents recycled as with the mammalian lipoproteins, lipophorin is a reusable lipid shuttle that delivers lipids to storage or peripheral tissues before returning for another cycle of loading and unloading.
When there is a demand for energy, as during hibernation or for flight, triacylglycerols in the fat bodies are mobilized by conversion to 1,2‑diacyl-sn-glycerols (rather than to free acids as in vertebrates), which are loaded onto the HDLp particles, decreasing their density and producing low-density lipophorin (LDLp). The increase in hydrophobicity of the particles is countered by the uptake of up to 16 molecules of the exchangeable apolipophorin III (akin to vertebrate apoproteins) from the haemolymph. When this lipoprotein reaches the flight muscles, the diacylglycerols are hydrolysed to free acids by the action of lipoprotein lipase, and a fatty acid-binding protein then facilitates fatty acid transport within the cell. The phospholipids are enriched in polyunsaturated fatty acids, which are transported in this form to the central nervous system and other tissues where they are released through lipophorin receptor (LpR)-mediated endocytosis of lipophorin. In contrast, the diacylglycerols contain mainly C14 fatty acids (14:0 and 14:1) and may be transferred to different sites, where they are taken up selectively.
Recommended Reading
- Ahotupa, M. Lipid oxidation products and the risk of cardiovascular diseases: role of lipoprotein transport. Antioxidants, 13, 512 (2024); DOI.
- Axmann, M., Plochberger, B., Mikula, M., Weber, F., Strobl, W.M. and Stangl, H. Plasma membrane lipids: an important binding site for all lipoprotein classes. Membranes, 11, 882 (2021); DOI.
- Borén, J., Taskinen, M.R. and Packard, C.J. Biosynthesis and metabolism of ApoB-containing lipoproteins. Annu. Rev. Nutr., 44, 179-204 (2024); DOI.
- Cho, K.H. The current status of research on high-density lipoproteins (HDL): a paradigm shift from HDL quantity to HDL quality and HDL functionality. Int. J. Mol. Sci., 23, 3967 (2022); DOI.
- Darabi, M. and Kontush, A. High-density lipoproteins (HDL): Novel function and therapeutic applications. Biochim. Biophys. Acta, Lipids, 1867, 159058 (2022); DOI.
- de Boer, J.F., Kuipers, F. and Groen, A.K. Cholesterol transport revisited: a new turbo mechanism to drive cholesterol excretion. Trends Endocrinol. Metab., 29, 123-133 (2018); DOI.
- Giammanco, A., Spina, R., Cefalù, A.B. and Averna, M. APOC-III: a gatekeeper in controlling triglyceride metabolism. Curr. Ather. Rep., 25, 67-76 (2023); DOI.
- Gogonea, V. Structural insights into high density lipoprotein: old models and new facts. Front. Pharmacol., 6, 318 (2016); DOI.
- Ke, L.Y., Law, S.H., Mishra, V.K., Parveen, F., Chan, H.C., Lu, Y.H. and Chu, C.S. Molecular and cellular mechanisms of electronegative lipoproteins in cardiovascular diseases. Biomedicines, 8, 550 (2020); DOI.
- Koerner, C.M., Roberts, B.S. and Neher, S.B. Endoplasmic reticulum quality control in lipoprotein metabolism. Mol. Cell. Endocrin., 498, 110547 (2019); DOI.
- Kuai, R., Li, D., Chen, Y.E., Moon, J.J. and Schwendeman, A. High-density lipoproteins: nature's multifunctional nanoparticles. ACS Nano, 10, 3015-3041 (2016); DOI.
- Musselman, L.P. and Kühnlein, R.P. Drosophila as a model to study obesity and metabolic disease. J. Exp. Biol., 221, jeb163881 (2018); DOI.
- Ong, K.-L., Cochran, B.J., Manandhar, B., Thomas, S. and Rye, K.-A. HDL maturation and remodelling. Biochim. Biophys. Acta, Lipids., 1867, 159119 (2022); DOI.
- Qu, J., Ko, C.W., Tso, P. and Bhargava, A. Apolipoprotein A-IV: a multifunctional protein involved in protection against atherosclerosis and diabetes. Cells, 8, 319 (2019); DOI.
- Raulin, A.C., Martens, Y.A. and Bu, G.J. Lipoproteins in the central nervous system: from biology to pathobiology. Annu. Rev. Biochem., 91, 731-759 (2022); DOI.
- Ridgway, N.D. and McLeod, R.S. (Editors) Biochemistry of Lipids, Lipoproteins and Membranes, 6th Edition. (Elsevier, Amsterdam) (2016) - several chapters - see Science Direct - there is now a 7th edition (2021).
- Rouland, A., Masson, D., Lagrost, L., Verges, B., Gautier, T. and Bouillet, B. Role of apolipoprotein C1 in lipoprotein metabolism, atherosclerosis and diabetes: a systematic review. Cardiovasc. Diabetol., 21, 272 (2022); DOI.
- Siri-Tarino, P.W. and Krauss, R.M. The early years of lipoprotein research: from discovery to clinical application. J. Lipid Res., 57, 1771-1777 (2016); DOI.
- Tasdighi, E., Adhikari, R., Almaadawy, O., Leucker, T.M. and Blaha, M.J. LP(a): structure, genetics, associated cardiovascular risk, and emerging therapeutics. Annu. Rev. Pharm. Toxic., 64, 135-157 (2024); DOI.
- Turri, M., Marchi, C., Adorni, M.P., Calabresi, L. and Zimetti, F. Emerging role of HDL in brain cholesterol metabolism and neurodegenerative disorders. Biochim. Biophys. Acta, Lipids, 1867, 159123 (2022); DOI.
- Yu, X.-H., Zhang, D.-W., Zheng, X.L. and Tang, C.-K. Cholesterol transport system: An integrated cholesterol transport model involved in atherosclerosis. Prog. Lipid Res., 73, 65-91 (2019); DOI.
- Zhang, R. and Zhang, K.Z. A unified model for regulating lipoprotein lipase activity. Trends Endocrinol. Metab., 35, 490-504 (2024); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: September 25th, 2024 | ||
