Triacylglycerols: 1. Structure and Composition
Nearly all the commercially important fats and oils of animal and plant origin contain mainly (>95%) the simple lipid class - triacylglycerols ('triglycerides'), which are chemically inert, highly hydrophobic and have a high energy density. This includes all the vegetable oils, such as those from corn (maize), olive, palm and sunflower, and animal fats, such as tallow, lard and butter, as well as manufactured products such as margarines. The more abundant animal triacylglycerols are depots fats (from adipose tissue) or milk fats, where their main function in vivo is as a store of energy (and of bioactive lipids), but it is increasingly being recognized that triacylglycerols in most tissues, but especially those in plasma or liver, have a more dynamic role in cellular metabolism. Similarly, seed oils serve as a source of energy and structural fatty acids for the developing plant embryo. In this web page, the fatty acid compositions of triacylglycerols together with their organization within molecules in various organisms are described. Two companion documents on this website are Triacylglycerols: Part 2. Biosynthesis and metabolism and Triacylglycerols: Part 3 - Regio- and stereospecific analysis procedures.
1.1. Introduction
In chemical terms, triacylglycerols consist of the trihydric alcohol glycerol esterified mainly with long-chain fatty acids (C14 to C22). When the two primary hydroxyl groups are esterified with different fatty acids, the resulting triacylglycerol can display chiral asymmetry and thus be optically active, although this is usually too low to be measured. The conventional D/L or R/S systems could designate such enantiomers without ambiguity with simple molecules, but problems arise in application to the complex range of molecular species of triacylglycerols found in nature. Such problems can be avoided if the stereochemistry of triacylglycerols and other glycerolipids is described by the "stereospecific numbering" (sn) system as recommended by a IUPAC-IUB commission (see our web page on Nomenclature for references).
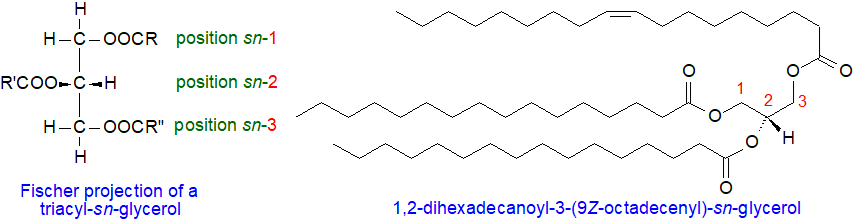 |
| Figure 1. Structures of triacyl-sn-glycerols. |
In a Fischer projection of a natural L-glycerol derivative as shown above, the secondary hydroxyl group is shown to the left of C-2; the carbon atom above this then becomes C-1 while that below becomes C-3, and the prefix sn is placed before the stem name of the compound. The term "triacyl-sn-glycerol" should then be used to designate the molecule, as it is technically accurate from the standpoint of systematic nomenclature and is essential for the stereospecific numbering system. The older term "triglyceride" is still in use in industrial and clinical settings, but it is not recommended for biosynthetic, metabolic and lipidomic studies, where comparisons with the structures of other glycerolipids are made. When the detailed stereochemistry is not specified, the primary hydroxy groups are often termed the α- and α′‑positions and the secondary, the β-position. A representative single molecular species 1,2‑dihexadecanoyl-3-(9Z-octadecenoyl)-sn-glycerol is illustrated.
Differences in the distributions of fatty acids among the positions of the glycerol moiety in triacylglycerols from natural fats and oils were first demonstrated systematically by enzymatic hydrolysis procedures, mainly pancreatic lipase hydrolysis for the analysis of the fatty acids of position sn-2 (regiospecific analysis), before complex stereospecific hydrolysis procedures were developed that permitted the distributions of fatty acids in all three positions to be determined (see Part 3 of this topic). Because of this historical development of the analytical methodology, there has been a tendency to assume that the composition of fatty acids esterified to the sole secondary hydroxyl group must have greater meaning than those of the two primary positions. It is certainly true that the composition of position sn‑2 is of great importance when triacylglycerols are consumed and digested by animals, since 2-monoacyl-sn-glycerols are then formed which can be absorbed by the intestines and utilized as such. On the other hand, the results of stereospecific analyses have shown that the compositions of all three positions in certain fats can be distinctive and can highlight crucial aspects of the biosynthetic processes.
The discussion that follows centres on the fatty acid compositions and the stereospecific distribution of fatty acids within triacyl-sn-glycerols with a few selected examples. Note that all data are presented as mol% not weight%. While a high proportion of recent analyses utilize mass spectrometric determinations of molecular species, regiospecific analysis only of triacylglycerols is possible by this means and the molecular species data are not easy to reproduce here in a simple tabulated form. Even a relatively simple natural triacylglycerol with only five different fatty acid constituents can in theory exist in the form of 125 molecular species, or 75 if enantiomers are ignored. Therefore, I need make no apology for using positional data from older publications for comparative purposes here.
1.2. Triacylglycerols from Seed Oils
 While seed and fruit oils within the plant
kingdom can contain a vast range of different fatty acids, those that are used most widely for food purposes tend to have
relatively simple compositions in which C18 unsaturated fatty acids, i.e., oleate and linoleate, are predominant.
Thus, olive oil contains over 70% of oleic acid, while safflower oil can contain a similar high proportion of linoleate,
which is also the major component of maize (corn) and soybean oils.
As linseed oil contains about 60% α-linolenic acid, which oxidizes readily, it is not suitable for food purposes although it has
many industrial uses.
For this reason, soybean oil, with 3 to 7% linolenic acid, is often hydrogenated before it is used as a food ingredient.
Rapeseed oil is available in two forms, i.e., with high or low erucic acid contents; the former has industrial value,
but it is banned from foods because of fears about the safety of erucic acid for human consumption.
Castor oil, with mainly industrial applications, contains up to 90% ricinoleic acid (see our web pages on
hydroxy fatty acids).
The so-called ‘tropical oils’, such as palm oil, contain higher amounts of saturated fatty acids than most other commercial oils,
while palm kernel and coconut oils are rich sources of medium-chain fatty acids.
Some representative analyses are listed in Table 1.
While seed and fruit oils within the plant
kingdom can contain a vast range of different fatty acids, those that are used most widely for food purposes tend to have
relatively simple compositions in which C18 unsaturated fatty acids, i.e., oleate and linoleate, are predominant.
Thus, olive oil contains over 70% of oleic acid, while safflower oil can contain a similar high proportion of linoleate,
which is also the major component of maize (corn) and soybean oils.
As linseed oil contains about 60% α-linolenic acid, which oxidizes readily, it is not suitable for food purposes although it has
many industrial uses.
For this reason, soybean oil, with 3 to 7% linolenic acid, is often hydrogenated before it is used as a food ingredient.
Rapeseed oil is available in two forms, i.e., with high or low erucic acid contents; the former has industrial value,
but it is banned from foods because of fears about the safety of erucic acid for human consumption.
Castor oil, with mainly industrial applications, contains up to 90% ricinoleic acid (see our web pages on
hydroxy fatty acids).
The so-called ‘tropical oils’, such as palm oil, contain higher amounts of saturated fatty acids than most other commercial oils,
while palm kernel and coconut oils are rich sources of medium-chain fatty acids.
Some representative analyses are listed in Table 1.
Table 1. Positional distributions of fatty acids (mol %) in triacyl-sn-glycerols of seed oils. |
|||||||
| Oil | Position | Fatty acid | |||||
|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | C20-C24 | ||
| Peanut | TG | 9 | 3 | 58 | 23 | 7 | |
| 1 | 14 | 5 | 59 | 19 | 4 | ||
| 2 | 2 | tr | 59 | 39 | 1 | ||
| 3 | 11 | 5 | 57 | 10 | 15 | ||
| Rapeseed* | TG | 3 | 2 | 26 | 17 | 10 | 43 |
| 1 | 4 | 2 | 23 | 11 | 53 | ||
| 2 | 1 | 37 | 36 | 6 | 6 | ||
| 3 | 4 | 3 | 17 | 4 | 20 | 70 | |
| Soybean | TG | 9 | 4 | 24 | 54 | 8 | |
| 1 | 14 | 6 | 23 | 48 | 9 | ||
| 2 | 1 | tr | 22 | 70 | 7 | ||
| 3 | 13 | 6 | 28 | 45 | 9 | ||
| Linseed | TG | 6 | 4 | 16 | 17 | 57 | |
| 1 | 10 | 6 | 15 | 16 | 53 | ||
| 2 | 2 | 1 | 16 | 21 | 60 | ||
| 3 | 6 | 4 | 17 | 13 | 59 | ||
| Maize (corn) | TG | 11 | 2 | 29 | 57 | 1 | |
| 1 | 1 | 3 | 28 | 50 | 1 | ||
| 2 | 18 | tr | 27 | 70 | 1 | ||
| 3 | 2 | 3 | 31 | 52 | 1 | ||
| Olive | TG | 10 | 2 | 76 | 10 | 1 | |
| 1 | 13 | 3 | 72 | 10 | 1 | ||
| 2 | 1 | 83 | 14 | 1 | |||
| 3 | 17 | 4 | 74 | 5 | |||
| Cacao butter | TG | 24 | 35 | 36 | 3 | tr | 1 |
| 1 | 34 | 50 | 12 | 1 | 1 | 1 | |
| 2 | 2 | 2 | 87 | 9 | |||
| 3 | 37 | 53 | 9 | tr | 2 | ||
| Palm | TG | 48 | 4 | 36 | 10 | ||
| 1 | 60 | 3 | 27 | 9 | |||
| 2 | 13 | tr | 68 | 18 | |||
| 3 | 72 | 8 | 14 | 3 | |||
| tr = trace (<0.5% ). * High erucic acid rapeseed oil.
TG = intact triacylglycerols Data from - Brockerhoff, H. and Yurkowski, M., J. Lipid Res., 7, 62-64 (1966); DOI. Christie, W.W. et al., Lipids, 68, 695-701 (1991); DOI. |
|||||||
As was well known from studies involving hydrolysis with pancreatic lipase, position sn-2 of the triacylglycerols of seed oils is greatly enriched in the polyunsaturated linoleic and linolenic acids, while saturated fatty acids are concentrated in the primary positions, and monoenoic acids are relatively evenly distributed. There are exceptions to these rules, and in cacao butter, oleic acid is present largely in position sn-2. While minor differences only in the distributions of saturated and monoenoic fatty acids between positions sn-1 and sn-3 have been observed, too few analyses have been published for definitive comment. Longer-chain fatty acids (C20 to C24) tend to be concentrated in the primary positions with some small preference for position sn-3. Although the average compositions of positions sn-1 and sn-3 may be similar, there can be greater differences in individual molecular species, as has been observed for olive oil (Santinelli, F. et al. J. Am. Oil Chem. Soc., 69, 552-556 (1992); DOI).
In those seed oils containing unusual fatty acids that have been subjected to stereospecific analysis, an allenic estolide was found entirely in position sn-3 in the triacyl-sn-glycerols of Sapium sebiferum, acetic acid appeared to be linked entirely to position sn-3 in Euonymus verrucosus, and much of the coriolic acid was found in position sn-3 in Monnina emarginata.
In summary, seed oils containing the usual range of saturated and unsaturated fatty acids tend to have the polyunsaturated components in position sn-2, but relatively little difference between the primary positions; less-common fatty acids tend to be concentrated in position sn-3. Only a few seed oils of commercial value have been analysed in this way, although many more have been subjected to regiospecific analysis and a vast amount of other compositional data are available in the scientific literature. I am not aware of comparable data from plant triacylglycerols other than seed oils or from those of algae.
1.3. Triacylglycerols from Animal Fats - Adipose Tissue
Much of the triacylglycerols in animal tissues, including some of the commercially important fats such as lard or tallow, are contained within various adipose tissue sites in cells known as adipocytes (or in lipid droplets within cells), where they serve mainly as an energy store, but also cushion tissues such as liver. The subcutaneous fats help to insulate animals, while fat stores in fish and marine animals help to maintain buoyancy. While some fatty acids are synthesised de novo in tissues (saturated and monoenoic acids), the composition of the triacylglycerols reflects that of the diet to an appreciable extent.
In terrestrial animals, the composition tends to be quite simple, with C16 (mainly 16:0) and C18 fatty acids predominating. All the essential fatty acids, such as linoleic and α-linolenic acids, must come from the diet of course, although polyunsaturated fatty acids tend to be relatively minor components of triacylglycerols in comparison to the complex lipids. Ruminant animals, such as the cow and sheep, have relatively saturated fats because the dietary unsaturated fatty acids are subjected to biohydrogenation in the rumen, a process that generates trans fatty acids as by-products. In addition, these animals end to have relatively higher concentrations of odd- and branched-chain fatty acids derived from the rumen microflora. Non-ruminant herbivores, such as the horse, can have appreciable amounts of linolenic acid from grass in their adipose tissue, while that of marine mammals is characterized by high concentrations of long-chain mono- and polyenoic fatty acids because of their diet of fish. Fish oils have their own section below.
Marked differences have been observed in the distributions of fatty acids among the three positions of the glycerol moiety in most species examined, and there are appreciable inter-species differences. A few representative results are listed in Table 2.
Table 2. Positional distributions of fatty acids (mol %) in triacyl-sn-glycerols of animal depot fats. |
||||||||
| Species | Position | Fatty acid | ||||||
|---|---|---|---|---|---|---|---|---|
| 14:0 | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | ||
| Human | TG | 5 | 24 | 7 | 8 | 46 | 7 | 1 |
| 1 | 4 | 42 | 3 | 15 | 27 | 6 | 1 | |
| 2 | 6 | 10 | 12 | 2 | 55 | 4 | 2 | |
| 3 | 4 | 19 | 6 | 6 | 57 | 11 | 1 | |
| Cattle | TG | 5 | 27 | 6 | 17 | 33 | 5 | 1 |
| 1 | 4 | 41 | 6 | 17 | 20 | 4 | 1 | |
| 2 | 9 | 17 | 6 | 9 | 41 | 5 | 1 | |
| 3 | 1 | 22 | 6 | 24 | 37 | 5 | 1 | |
| Sheepa | TG | 3 | 22 | 2 | 35 | 36 | 2 | |
| 1 | 1 | 35 | 2 | 47 | 4 | - | ||
| 2 | 4 | 14 | 2 | 15 | 52 | 5 | ||
| 3 | 3 | 16 | 1 | 42 | 26 | 2 | ||
| Pig | TG | 2 | 27 | 3 | 13 | 45 | 9 | |
| 1 | 1 | 10 | 2 | 30 | 51 | 6 | ||
| 2 | 4 | 72 | 5 | 2 | 13 | 3 | ||
| 3 | - | tr | 2 | 7 | 70 | 18 | ||
| Rat | TG | 2 | 23 | 5 | 6 | 35 | 26 | 1 |
| 1 | 2 | 32 | 5 | 9 | 32 | 15 | 1 | |
| 2 | 1 | 10 | 4 | 1 | 37 | 45 | 1 | |
| 3 | 2 | 27 | 5 | 7 | 37 | 17 | 1 | |
| Rabbit | TG | 3 | 28 | 9 | 3 | 29 | 20 | 4 |
| 1 | 3 | 34 | 9 | 6 | 25 | 14 | 2 | |
| 2 | 6 | 25 | 12 | 1 | 26 | 23 | 5 | |
| 3 | 1 | 24 | 7 | 3 | 35 | 22 | 5 | |
| Chicken | TG | 1 | 30 | 6 | 6 | 45 | 11 | 1 |
| 1 | 1 | 47 | 7 | 8 | 31 | 5 | 1 | |
| 2 | tr | 13 | 5 | 6 | 55 | 19 | 1 | |
| 3 | 1 | 31 | 7 | 3 | 49 | 8 | 1 | |
| a Results are listed for cis-18:1 isomers only; trans-18:1 was present in positions
sn-1, sn-2 and sn-3 as 5, 2 and 6 %, respectively. tr = trace (<0.5%). TG = intact triacylglycerols Data from – Christie, W.W. et al., Lipids, 6, 854-856 (1971); DOI, Brockerhoff, H. et al., Biochim. Biophys. Acta, Lipids, 116, 67-72 (1966); DOI. Christie, W.W. and Moore, J.H. J. Sci. Food. Agric., 22, 120-124 (1971); DOI. Biochim. Biophys. Acta, Lipids, 210, 46-56 (1970); DOI. J. Sci. Food. Agric., 23, 73-77 (1972); DOI. |
||||||||
 For most species,
saturated fatty acids are found predominantly in position sn-1, although appreciable amounts of oleic acid are usually present.
Position sn-2 tends to contain mainly unsaturated fatty acids, mainly linoleic acid, although some of the shorter-chain
fatty acids can accumulate here in some instances.
There is some preference for the longer-chain fatty acids to be located in position sn-3.
The main exception to these rules is the pig and related species where palmitic acid can comprise more than 70% of the fatty acids
in position sn-2, while of equal interest from a biosynthetic standpoint, most of the stearic acid (75% of that in the tissue)
is in position sn-1 and position sn-3 contains more than 70% of oleic acid.
Although the absolute fatty acid compositions of adipose tissue at various sites in an animal can vary somewhat
(subcutaneous fats tend to contain more unsaturated fatty acids than internal depot fats),
the proportional distributions of each fatty acid among the three positions does not vary significantly in
any of these tissues in either in the pig or the sheep.
For most species,
saturated fatty acids are found predominantly in position sn-1, although appreciable amounts of oleic acid are usually present.
Position sn-2 tends to contain mainly unsaturated fatty acids, mainly linoleic acid, although some of the shorter-chain
fatty acids can accumulate here in some instances.
There is some preference for the longer-chain fatty acids to be located in position sn-3.
The main exception to these rules is the pig and related species where palmitic acid can comprise more than 70% of the fatty acids
in position sn-2, while of equal interest from a biosynthetic standpoint, most of the stearic acid (75% of that in the tissue)
is in position sn-1 and position sn-3 contains more than 70% of oleic acid.
Although the absolute fatty acid compositions of adipose tissue at various sites in an animal can vary somewhat
(subcutaneous fats tend to contain more unsaturated fatty acids than internal depot fats),
the proportional distributions of each fatty acid among the three positions does not vary significantly in
any of these tissues in either in the pig or the sheep.
A variety of dietary factors can influence the fatty acid compositions and thence the structures of depot fat triacylglycerols, not least the composition of the diet. Few systematic studies of the relationship between triacylglycerol composition and structure within a single species have been performed, and in the example below, the pig was the experimental animal with the data for palmitic acid selected for illustrative purposes in Figure 2.
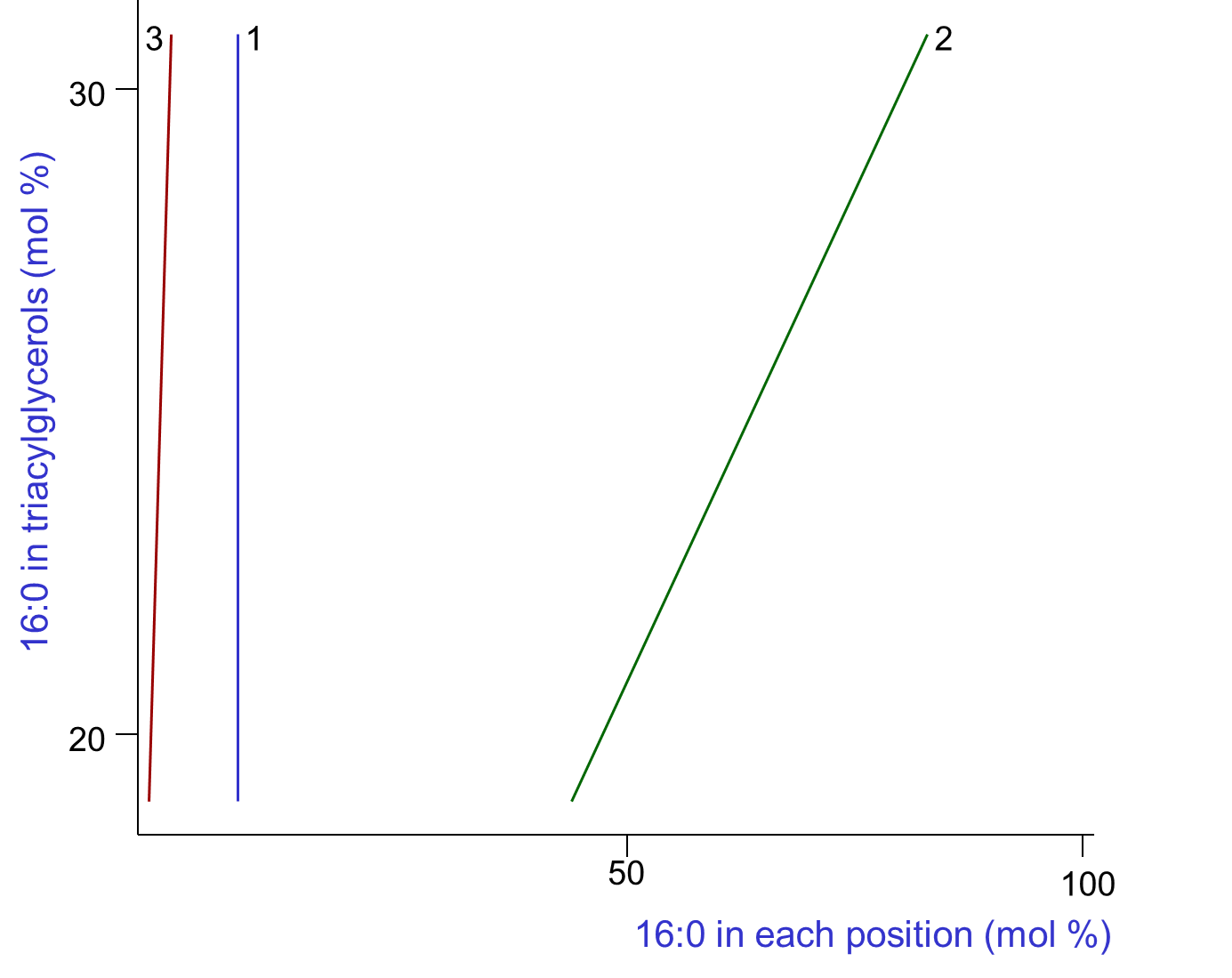 |
| Figure 2. Variation of the composition of palmitic acid in positions sn-1, sn-2 and sn-3 of the triacylglycerols of pig adipose tissue with changes in overall composition (Christie, W.W. and Moore, J.H. Lipids, 5, 921-928 (1970); DOI). |
As the proportion of palmitic acid in the triacylglycerols varied between 18 and 33%, the amount in position sn-1 remained constant at about 10%, that in position sn-3 increased linearly but relatively slowly from 2 to 4%, while that in position sn-2 was affected most and increased rapidly from less than 50% to more than 70%. Over the range of compositions available for study, the amounts of all the fatty acids varied in a characteristic manner that was linear in each of the three positions, though data for 16:0 only are illustrated.
In experiments with rat adipocytes in vitro, stereospecific analyses demonstrated that fatty acids of extracellular origin were esterified to each position of the triacyl-sn-glycerols in similar, but not identical, proportions to the natural distributions, while most of the oleic acid synthesised in the tissue by desaturation of exogenous stearic acid was found in position sn-3. In contrast, fatty acids synthesised de novo were esterified only to positions sn-1 and sn-2 (Henderson, R.J. et al. Biochim. Biophys. Acta, Lipids, 574, 8-17 (1979); DOI). This phenomenon has now been explained by differences in the membrane location of the diacylglycerol acyl transferase (DGAT1 form) in cells.
A freeze-tolerant insect Eurosta solidaginis produces a high proportion of acetylated triacylglycerols in winter for storage purposes, and presumably, these remain liquid at low temperatures when the insect is dormant so remain available for energy purposes. Aphids secrete distinctive triacylglycerols onto their external surfaces with myristic acid in the primary positions and high concentrations of short-chain fatty acids, including sorbic acid (2,4-hexadienoic acid), in position sn‑2.
1.4. Triacylglycerols from Animal Fats – Milk Fat
 Milk
fats are the only animal lipids that have evolved to serve as a food.
In these, the range of fatty acids is more extensive than in adipose tissue with often a higher proportion of
short- and medium-chain fatty acids, which are not used as such for structural purposes but provide a rapid source of energy.
Ruminants, such as the cow, have a range of saturated fatty acids from butyric upwards (indeed even acetate has been found in esterified form),
and there are relatively low amounts only of polyunsaturated fatty acids because of biohydrogenation in the rumen.
In other species, the compositions are less extreme, and in human milk, 12:0 and 14:0 fatty acids are more abundant
than in other tissues, while the linoleic acid concentration varies between 10 and 20% depending on diet.
Milk
fats are the only animal lipids that have evolved to serve as a food.
In these, the range of fatty acids is more extensive than in adipose tissue with often a higher proportion of
short- and medium-chain fatty acids, which are not used as such for structural purposes but provide a rapid source of energy.
Ruminants, such as the cow, have a range of saturated fatty acids from butyric upwards (indeed even acetate has been found in esterified form),
and there are relatively low amounts only of polyunsaturated fatty acids because of biohydrogenation in the rumen.
In other species, the compositions are less extreme, and in human milk, 12:0 and 14:0 fatty acids are more abundant
than in other tissues, while the linoleic acid concentration varies between 10 and 20% depending on diet.
Severe technical problems were encountered in the stereospecific analysis of milk triacyl-sn-glycerols from ruminants because of the presence of short-chain fatty acids, which give rise to difficulties in the isolation of the required partially hydrolysed intermediates. To overcome the problem, Breckenridge et al. isolated fractions enriched in either long-chain or short-chain components by means of molecular distillation or thin-layer chromatography and subjected these separately to stereospecific analysis, combining the results at the end of the procedure. The data showed unequivocally that cows' milk is one of the most asymmetric of animal fats, containing all the butyric acid and most of the hexanoic acid in position sn-3. Some representative results for the cow, humans and other species are listed in Table 3.
Table 3. The composition of the fatty acids esterified to each position of the triacyl-sn-glycerols in the milk fats of various species. |
|||||||||||||
| Species | Position | Fatty acid | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4:0 | 6:0 | 8:0 | 10:0 | 12:0 | 14:0 | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | ||
| Cow | TG | 12 | 5 | 2 | 4 | 4 | 11 | 24 | 2 | 7 | 24 | 3 | |
| 1 | - | 1 | 1 | 2 | 5 | 10 | 34 | 2 | 10 | 30 | 2 | ||
| 2 | - | 1 | 1 | 3 | 6 | 18 | 32 | 4 | 10 | 19 | 4 | ||
| 3 | 35 | 13 | 4 | 6 | 1 | 6 | 5 | 1 | 1 | 23 | 2 | ||
| Human | TG | tr | 3 | 26 | 27 | 6 | 7 | 36 | 11 | 1 | |||
| 1 | tr | 1 | 3 | 16 | 4 | 15 | 46 | 11 | tr | ||||
| 2 | tr | 2 | 7 | 58 | 5 | 3 | 13 | 7 | 1 | ||||
| 3 | 1 | 6 | 7 | 6 | 8 | 2 | 50 | 15 | 1 | ||||
| Rat | TG | 6 | 19 | 14 | 12 | 21 | 2 | 3 | 13 | 10 | 1 | ||
| 1 | 3 | 10 | 10 | 10 | 20 | 2 | 5 | 24 | 14 | 1 | |||
| 2 | 6 | 20 | 16 | 18 | 29 | 2 | 1 | 3 | 5 | 1 | |||
| 3 | 10 | 26 | 15 | 9 | 13 | 2 | 2 | 12 | 12 | 1 | |||
| Pig | TG | 4 | 32 | 9 | 5 | 39 | 10 | 1 | |||||
| 1 | 2 | 22 | 7 | 7 | 50 | 11 | 1 | ||||||
| 2 | 7 | 58 | 11 | 1 | 15 | 8 | 1 | ||||||
| 3 | 4 | 15 | 10 | 6 | 52 | 12 | 2 | ||||||
| tr = trace (< 0.5 %). TG = intact triacylglycerols. Data from - Christie, W.W. and Moore, J.H. Biochim. Biophys. Acta, Lipids, 210, 46-56 (1970); DOI. Christie, W.W. J. Dairy Res., 52, 219-222 (1985); DOI. Christie, W.W. and Clapperton, J.L. J. Soc. Dairy Technol., 35, 22-24 (1982); DOI. Breckenridge, W.C. et al., Canad. J. Biochem., 47, 761-769 (1969); DOI. |
|||||||||||||
As the overall fatty acid compositions of the triacyl-sn-glycerols are very different for each species, depending both on the diet and the nature of the fatty acids synthesised in the mammary gland, similarities are not immediately apparent, but a close perusal can reveal certain common features for the longer-chain fatty acids. For most species, much of the palmitic acid is concentrated in position sn-2, the only known exception being the primitive monotreme, the echidna. Other than in the pig (and elephant), a structure with such a high content of palmitic acid in position sn-2 is not common in animal triacylglycerols. Myristic acid and the medium-chain fatty acids, together with palmitic acid, are found in the greatest concentration in position sn‑2, but stearic acid is concentrated in the primary positions, mainly position sn-1. The unsaturated fatty acids tend to be present in the greatest concentrations in positions sn-1 and sn-3. Human milk triacylglycerols are enriched in palmitic acid in position sn-2, which is reported to aid digestion, and commercial infant formulae are engineered to have this type of composition.
Milk fat is produced in the form of cytoplasmic droplets, mainly composed of triacylglycerols, and during secretion from the endoplasmic reticulum, these are coated with a monolayer of phospholipids derived from this membrane. Later, when secreted from the cell, these droplets bind to the apical plasma membrane, to form an outer bilayer to the milk fat globule membrane that contains many different complex polar lipids and proteins. This three-layer membrane contains 2–6% of the lipids in milk, adding to the nutritional value, and it is a natural emulsifier that prevents the fat from coalescing.
1.5. Triacylglycerols from Animal Fats – Other Tissues
The fatty acid compositions of triacyl-sn-glycerols of animal tissues other than adipose tissue tend to resemble those of the latter, but the stereospecific distributions can differ. Some representative analyses are listed in Table 4.
Table 4. Positional distributions of fatty acids (mol %) in triacyl-sn-glycerols from animal tissues other than depot fats and milk. |
||||||
| Position | Fatty acid | |||||
|---|---|---|---|---|---|---|
| Source | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | |
| Sheep livera | TG | 26 | 3 | 16 | 43 | 5 |
| 1 | 47 | 2 | 17 | 21 | 1 | |
| 2 | 14 | 3 | 18 | 55 | 8 | |
| 3 | 16 | 3 | 13 | 54 | 5 | |
| Sheep plasma | TG | 28 | 2 | 22 | 31 | 2 |
| 1 | 34 | 2 | 33 | 21 | 2 | |
| 2 | 38 | 3 | 6 | 33 | 1 | |
| 3 | 11 | 2 | 28 | 40 | 2 | |
| Sheep adrenals | TG | 23 | 2 | 16 | 32 | 3 |
| 1 | 33 | 2 | 31 | 28 | 1 | |
| 2 | 41 | 4 | 4 | 26 | 3 | |
| 3 | 4 | 1 | 15 | 48 | 4 | |
| Chicken plasma | TG | 29 | 5 | 5 | 50 | 11 |
| 1 | 74 | 6 | 4 | 13 | 1 | |
| 2 | 4 | 3 | 2 | 60 | 31 | |
| 3 | 8 | 5 | 8 | 76 | 2 | |
| Chicken egg | TG | 29 | 5 | 7 | 49 | 10 |
| 1 | 71 | 5 | 4 | 17 | 2 | |
| 2 | 4 | 3 | 3 | 63 | 26 | |
| 3 | 12 | 6 | 14 | 67 | 1 | |
| a sheep tissues contain appreciable amounts of minor components not listed here
(e.g. odd- and branched-chain). TG = intact triacylglycerols. Data from - Christie, W.W. and Noble, R.C. J. Sci. Food. Agric., 35, 617-624 (1984); DOI. Christie, W.W. and Moore, J.H. J. Sci. Food. Agric., 22, 120-124 (1971); DOI. Comp. Biochem. Physiol., 41B, 287-295 (1972); DOI. Biochim. Biophys. Acta, Lipids, 218, 83-88 (1970); DOI. Data for rat liver triacylglycerols are listed here.., and for human VLDL, not listed here but published by Agren, J.J., Ravandi, A., Kuksis, A. and Steiner, G. Eur. J. Biochem., 269, 6223-6232 (2002); DOI. |
||||||
 The pig
and sheep have probably been most studied.
With the latter in the liver triacylglycerols, for example, the stearic acid is distributed approximately equally between the three positions,
and the oleic acid is present in high concentrations in positions sn-2 and sn-3, thus differing from the depot fats (Table 2).
The distinctive feature of sheep plasma triacylglycerols is a high concentration of palmitic acid in position sn-2, and in this they
resemble those of the lymph, from which they are derived biosynthetically.
In most other tissues of the sheep, the triacylglycerols resemble those of adipose tissue, although in adrenal glands from which adhering
adipose tissue had been carefully removed, triacylglycerols containing a high proportion of palmitic acid in position sn-2 were again found
with long-chain polyunsaturated fatty acids (10% or more, but not listed here) in positions sn-2 and sn-3.
It is possible that triacylglycerols of this kind are more widespread than has been thought, and further research may reveal more
such distributions.
The pig
and sheep have probably been most studied.
With the latter in the liver triacylglycerols, for example, the stearic acid is distributed approximately equally between the three positions,
and the oleic acid is present in high concentrations in positions sn-2 and sn-3, thus differing from the depot fats (Table 2).
The distinctive feature of sheep plasma triacylglycerols is a high concentration of palmitic acid in position sn-2, and in this they
resemble those of the lymph, from which they are derived biosynthetically.
In most other tissues of the sheep, the triacylglycerols resemble those of adipose tissue, although in adrenal glands from which adhering
adipose tissue had been carefully removed, triacylglycerols containing a high proportion of palmitic acid in position sn-2 were again found
with long-chain polyunsaturated fatty acids (10% or more, but not listed here) in positions sn-2 and sn-3.
It is possible that triacylglycerols of this kind are more widespread than has been thought, and further research may reveal more
such distributions.
 Other unusual triacylglycerols of animal origin are
found in the tissues of the chicken.
In the plasma triacylglycerols, palmitic acid comprises over 70% of the fatty acids of
position sn-1 with relatively small amounts in positions sn-2 and sn-3,
while oleic acid comprised 60% of the fatty acids in position sn-2 and more than 70% of that in position sn‑3.
Virtually identical structures were found in the ovarian follicles and in the egg, suggesting a common biosynthetic origin.
It should be recognized that structural analyses of lipids can rarely be used to prove the existence of a biosynthetic pathway,
but they can provide valuable pointers to the biochemist to potentially productive experimental approaches.
Other unusual triacylglycerols of animal origin are
found in the tissues of the chicken.
In the plasma triacylglycerols, palmitic acid comprises over 70% of the fatty acids of
position sn-1 with relatively small amounts in positions sn-2 and sn-3,
while oleic acid comprised 60% of the fatty acids in position sn-2 and more than 70% of that in position sn‑3.
Virtually identical structures were found in the ovarian follicles and in the egg, suggesting a common biosynthetic origin.
It should be recognized that structural analyses of lipids can rarely be used to prove the existence of a biosynthetic pathway,
but they can provide valuable pointers to the biochemist to potentially productive experimental approaches.
Many types of cells contain lipid droplets enriched in triacylglycerols in the cytoplasm, and these are usually associated with a suite of proteins or enzymes that respond to physiological stimuli to release the fatty acid components (see Part 2). Such triacylglycerol stores are sometimes enriched in arachidonic acid for subsequent eicosanoid metabolism, and in mast cells and macrophages, triacylglycerols contain 45 and 22%, respectively, of the total cellular arachidonate (no stereo- or regio-specific analyses appear to be available). Human red blood cells, in contrast, have a small amount only of triacylglycerols, and these are enriched in saturated and monoenoic fatty acids with a relatively low polyunsaturated content.
1.6. Triacylglycerols from Fish Oils
The fatty acid compositions of triacylglycerols of fish oils reflect their diet and usually comprise high concentrations of long-chain monoenoic and polyunsaturated fatty acids, especially those of the omega-3 biosynthetic family (20:5, 22:5 and 22:6). The triacyl-sn-glycerols in depot fats from fish and other marine animals have been subjected to stereospecific analysis, and some typical results are listed in Table 5.
Table 5. Positional distributions of fatty acids (mol %) in triacyl-sn-glycerols of fish oils. |
||||||||||||
| Species | Position | Fatty acid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14:0 | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 20:1 | 22:1 | 20:5 | 22:5 | 22:6 | ||
| Herring | TG | 7 | 12 | 9 | 1 | 11 | 2 | 17 | 23 | 9 | 2 | 5 |
| 1 | 6 | 12 | 13 | 1 | 16 | 3 | 25 | 14 | 3 | 1 | 1 | |
| 2 | 10 | 17 | 10 | 1 | 10 | 3 | 6 | 5 | 18 | 3 | 13 | |
| 3 | 4 | 7 | 5 | 1 | 8 | 1 | 20 | 50 | 4 | 1 | 1 | |
| Mackerel | TG | 6 | 14 | 7 | 2 | 17 | 2 | 11 | 16 | 9 | 2 | 9 |
| 1 | 6 | 15 | 11 | 3 | 21 | 2 | 8 | 18 | 5 | 1 | 2 | |
| 2 | 10 | 21 | 6 | 1 | 9 | 1 | 5 | 5 | 12 | 3 | 20 | |
| 3 | 2 | 5 | 4 | 2 | 21 | 2 | 19 | 24 | 10 | 1 | 5 | |
| Skate | TG | 2 | 13 | 8 | 2 | 23 | 1 | 13 | 9 | 7 | 3 | 18 |
| 1 | 2 | 19 | 12 | 5 | 30 | 1 | 12 | 8 | 4 | 1 | 5 | |
| 2 | 3 | 15 | 7 | 1 | 9 | 1 | 8 | 5 | 6 | 7 | 37 | |
| 3 | 1 | 6 | 6 | 1 | 28 | 2 | 19 | 11 | 11 | 2 | 11 | |
| Cod | TG | 6 | 13 | 13 | 3 | 20 | 2 | 12 | 6 | 12 | 2 | 9 |
| 1 | 6 | 15 | 14 | 6 | 28 | 2 | 12 | 6 | 2 | 1 | 1 | |
| 2 | 8 | 16 | 12 | 1 | 9 | 2 | 7 | 5 | 12 | 3 | 20 | |
| 3 | 4 | 7 | 14 | 1 | 23 | 2 | 17 | 7 | 13 | 1 | 6 | |
| TG = intact triacylglycerols. Data from Brockerhoff, H., Hoyle, R.J., Hwang, P.C. and Litchfeld, C. Lipids, 3, 24-29 (1968); DOI. |
||||||||||||
Myristic, palmitic and palmitoleic acids are preferentially esterified to positions sn-1 and sn-3, together with oleic and longer-chain monoenoic fatty acids, with a tendency for a higher proportion to be in position sn-3 as the chain-length increases. In position sn-2, there is the greatest concentration of polyunsaturated fatty acids, with substantial amounts also being found in position sn-3. There are significant differences in triacyl-sn-glycerol structures between those of fish and of marine mammals that consume fish.
1.7. Triacylglycerols of Bacteria and Yeast
Triacylglycerols are common in eukaryotic organisms such as yeasts, moulds and fungi, but among the prokaryotes only species from the Actinomycetes group, including some of the human mycobacterial pathogens, accumulate triacylglycerols to a significant extent. As in eukaryotes, triacylglycerols appear to function as a reserve of fatty acyl groups, and they occur as cytoplasmic inclusions or lipid droplets within the organisms. Those from a few species only have been subjected to stereospecific analyses, and the fatty acid compositions of each are very different, so no general conclusions can be drawn. Three sets of data are listed in Table 6.
Table 6. Positional distributions of fatty acids (mol %) in triacyl-sn-glycerols of bacteria and yeast. |
|||||||||
| Species | Position | Fatty acid | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 14:0 | 16:0 | 16:1 | 17:0 | 18:0 | 18:1 | 18:2 | Other | ||
| M. smegmatis | TG | 3 | 24 | 10 | 1 | 10 | 29 | 22 | |
| 1 | 1 | 8 | 9 | tr | 7 | 60 | 10 | ||
| 2 | 7 | 57 | 13 | 2 | 6 | 9 | 4 | ||
| 3 | 1 | 7 | 7 | tr | 16 | 18 | 51 | ||
| R. opacus | TG | 4 | 26 | 10 | 13 | 3 | 22 | - | 22 |
| 1 | 12 | 18 | 7 | 12 | 6 | 24 | - | 20 | |
| 2 | 11 | 59 | tr | 18 | tr | tr | - | 12 | |
| 3 | - | - | 21 | 7 | 5 | 42 | - | 34 | |
| L. lipoferus | TG | 3 | 15 | 8 | 4 | 62 | 8 | ||
| 1 | 3 | 14 | 8 | 4 | 61 | 10 | |||
| 2 | - | 1 | 2 | - | 88 | 9 | |||
| 3 | 6 | 29 | 13 | 9 | 37 | 6 | |||
| tr = trace (<0.5%). TG = intact triacylglycerols.
Other = branched, odd- or longer-chain. Data from - Walker, R.W. et al. Lipids, 5, 684-691 (1970); DOI. Wältermann, M. et al. Microbiology, 146, 1143-1149 (2000); DOI. Haley, J.E. and Jack, R.C. Lipids, 9, 679-681 (1974); DOI. |
|||||||||
In the triacyl-sn-glycerols of Mycobacterium smegmatis, oleic acid is the main component of position sn-1, palmitic acid is the main component of position sn‑2, while C18 and longer-chain fatty acids are the principal constituents of position sn-3; indeed 90% of the 24:0 is in position sn-3. Although the fatty acid composition of Rhodococcus opacus is very different from this, the distribution of fatty acids is comparable in that saturated and shorter-chain fatty acids are concentrated in position sn-2. In the triacylglycerols of the yeast Lipomyces lipoferus, oleic acid is the main fatty acid in positions sn‑1 and sn‑2, while much of the palmitic, palmitoleic and stearic acids are found in position sn-3.
1.8. Analysis
A variety of chromatographic and spectrometric methods are available for the analysis of molecular species of triacylglycerols, while enzymatic hydrolysis with lipases such as pancreatic lipase can be used in the determination of the composition of position sn-2. In contrast, stereospecific determination of the distributions of fatty acids in all three positions of the glycerol moiety remains a substantial technical challenge, and only a few research groups appear to have retained the necessary expertise (see Part 3). Modern mass spectrometric methods are being used increasingly for determination of molecular species compositions in triacylglycerols and to distinguish between the compositions of position sn-2 and positions 1/3, but they do not distinguish between the two primary positions, i.e., they enable regiospecific but not stereospecific analysis. While an enormous amount of invaluable data from analyses of molecular species are available in the scientific literature from lipidomic studies, it tends to be unwieldy and not easily presented in tabulated form for comparison purposes here.
Other web pages on this site dealing with triacylglycerols are Triacylglycerols: Part 2 - their biosynthesis and metabolism and Triacylglycerols: Part 3 - regio- and stereospecific analysis procedures.
Recommended Reading
- Alvarez, H. and Steinbüchel, A. Triacylglycerols in prokaryotic microorganisms. Appl. Microbiol. Biotechn., 60, 367-376 (2002); DOI.
- Andrikopoulos, N.K. Triglyceride species compositions of common edible vegetable oils and methods used for their identification and quantification. Food Rev. Int., 18, 71-102 (2002); DOI.
- Breckenridge, W.C. Stereospecific analysis of triacylglycerols. In: 'Handbook of Lipid Research. Vol. 1. Fatty Acids and Glycerides', pp. 197-232 (edited by A. Kuksis, Plenum Press, New York) (1978).
- Christie, W.W. The positional distributions of fatty acids in triglycerides. In: The Analysis of Oils and Fats, pp. 313-339 (edited by R.J. Hamilton and J.B. Rossell, Elsevier Applied Science, London) (1986).
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Christie, W.W. The composition and structure of milk lipids. In: Advanced Dairy Chemistry - 2: Lipids (Second Edition), pp. 1-36 (edited by P.F. Fox, Chapman & Hall, London) (1995).
- Gunstone, F.D. and Harwood, J.L. Occurrence and characteristics of oils and fats. In: The Lipid Handbook. (Third Edition), pp. 37-141 (edited by F.D. Gunstone, J.L. Harwood and A.J. Dijkstra, Chapman & Hall, London) (2007) - see CRC Press.
- Kalpio, M., Linderborg, K.M., Fabritius, M., Kallio, H. and Yang, B.R. Strategy for stereospecific characterization of natural triacylglycerols using multidimensional chromatography and mass spectrometry. J. Chromatogr. A, 1641, 461992 (2021); DOI.
- Kuksis, A. Analysis of positional isomers of glycerolipids by non-enzymatic methods. In: Advances in Lipid Research - Three, pp. 1-36 (edited by W.W. Christie, Oily Press, Dundee) (1996).
- Laakso, P. Analysis of triacylglycerols: approaching the molecular composition of natural mixtures. Food Rev. Int., 12, 199-250 (1996); DOI.
- Lange, M., Angelidou, G., Ni, Z., Criscuolo, A., Schiller, J., Blüher, M. and Fedorova, M. AdipoAtlas: A reference lipidome for human white adipose tissue. Cell Rep. Med., 2, 100407 (2021); DOI.
- Ohlrogge, J., Thrower, N., Mhaske, V., Stymne, S., Baxter, M., Yang, W., Liu, J., Shaw, K., Shorrosh, B., Zhang, M. and Wilkerson, C. PlantFAdb: a resource for exploring hundreds of plant fatty acid structures synthesized by thousands of plants and their phylogenetic relationships. Plant J., 96, 1299-1308 (2018); DOI.
- Pan, J.Y., Chen, M.Q., Li, N., Han, R.W., Yang, Y.X., Zheng, N., Zhao, S.G. and Zhang, Y.D. Bioactive functions of lipids in the milk fat globule membrane: a comprehensive review. Foods, 12, 3755 (2023); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: October 2nd, 2024 | ||
