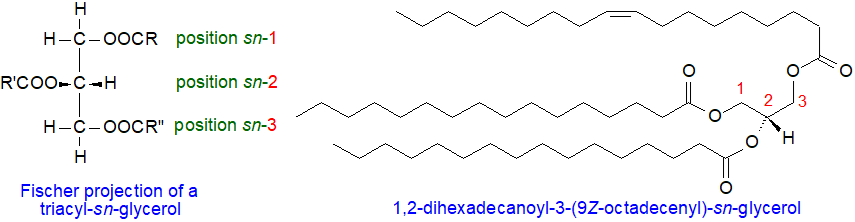
Triacylglycerols 3. Regio- and Stereospecific
Analysis of Triacyl-sn-glycerols
The compositions of the fatty acids linked to the different positions of the glycerol moiety of triacylglycerols of animals, plants and microorganisms can be very different. Position 2 is of course unique in that it is a secondary hydroxyl group, but it is not always recognized that the two primary positions are different stereochemically as there is a centre of asymmetry in the glycerol moiety. Indeed, biosynthetic mechanisms ensure that natural triacylglycerols invariably exist in enantiomeric forms. The positions are defined by a 'stereospecific numbering' (sn) system as sn-1, sn-2 and sn-3 from a Fischer projection, as defined by a IUPAC-IUB commission, and in natural triacyl-sn-glycerols, each can have a distinctive and often characteristic fatty acid composition. Analytical determination of these distributions is an invaluable tool in studies of the physical properties, biosynthesis and metabolism of triacylglycerols, and of their functions in tissues.
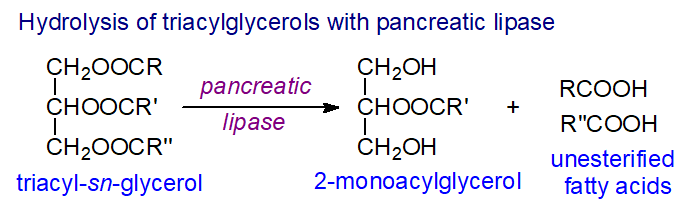
Hydrolysis by the enzyme pancreatic lipase is a relatively simple method for the determination of the composition of position 2, i.e., for regiospecific analysis of triacylglycerols. This can also be accomplished by a chemical procedure, modern mass spectrometry methods and by nuclear magnetic resonance spectroscopy.
However, no lipase has yet been identified with sufficient specificity to be of practical value to determine the compositions of positions sn‑1 and sn-3 of triacylglycerols. These can only be obtained by stereospecific analysis procedures with degradative and synthetic steps, followed by enzymatic hydrolysis and chromatographic separation of the products, including methods employing chiral chromatography, as reviewed by Kuksis and Itabashi [1,2]. Such methods have been used to determine the structures of many natural triacyl-sn-glycerols, and some surprisingly asymmetric fats have been found, with first prize going to milk fat in which all the short-chain fatty acids (butyric (4:0) and hexanoic (6:0) acids) are concentrated in position sn-3 with none in positions sn-1 and 2. Such data are discussed in greater detail for representative natural samples in our web page on Triacylglycerol compositions.
The following account is intended simply as a description of the principles of the main methods; it is not at all comprehensive, and a few key references only are listed (apologies to innumerable scientists who have worked on this topic).
3.1. Regiospecific Analysis of Triacyl-sn-glycerols by Lipase Hydrolysis
The composition of position sn-2 of triacylglycerols can be determined by incubating them with the enzyme pancreatic lipase (porcine) in an appropriate buffer. The fatty acids are hydrolysed from the primary positions leaving a 2-monoacyl-sn-glycerol, which can be isolated by thin-layer chromatography (TLC) or high-performance liquid chromatography (HPLC) for determination of its fatty acid composition, usually after trans-methylation for analysis by gas chromatography (GC). Pig pancreatin, a powder obtained by dehydrating and defatting pig pancreas with acetone and diethyl ether, is the most widely used source of the enzyme; it has no bias towards either of the primary positions, it is stable for long periods of time and is readily available from suppliers of biochemicals.
All straight-chain saturated fatty acids in the normal chain-length range and most mono-, di- and trienoic acids are apparently hydrolysed from the primary positions of triacylglycerols at about the same rate, although the ester bonds of polyunsaturated fatty acids such as docosahexaenoic (e.g., in fish oils), trans-3-hexadecenoic (e.g., from some plant sources), γ-linolenic acid and phytanic acid to glycerol are hydrolysed more slowly, probably as a result of steric hindrance caused by the proximity of substituent groups to the ester bonds. In addition, the enzyme hydrolyses triacylglycerol molecules that contain short-chain fatty acids more rapidly than molecules containing only longer-chain fatty acids. With a triacylglycerol such as 1-butyro-2,3-dipalmitin, both the fatty acids on the primary positions were found to be hydrolysed at about the same rate but faster than from tripalmitin. Fortunately, when the enzyme is used with triacylglycerols containing a more normal range of fatty acids, little acyl specificity is observed, and the 2-monoacylglycerols produced can be considered in practice to be representative of those in the native triacylglycerols.
 Calcium
ions are essential and bile salts helpful for the reaction, and it is necessary that the triacylglycerols be well dispersed by vigorous shaking,
as they must be in a micellar form for hydrolysis to occur.
For this reason, methyl oleate or hexane have sometimes been added as carriers to relatively saturated fats with high melting-points
(alternatively pre-incubation at 42°C for 5 min has been recommended).
In structural studies, the concentrations of the various cations, bile salts and the enzyme,
the pH of the buffer and the temperature are adjusted to their optima so that an appreciable degree of hydrolysis
(50-60% is sufficient) occurs in a short time.
Undesirable side reactions such as acyl migration are thereby minimized.
Calcium
ions are essential and bile salts helpful for the reaction, and it is necessary that the triacylglycerols be well dispersed by vigorous shaking,
as they must be in a micellar form for hydrolysis to occur.
For this reason, methyl oleate or hexane have sometimes been added as carriers to relatively saturated fats with high melting-points
(alternatively pre-incubation at 42°C for 5 min has been recommended).
In structural studies, the concentrations of the various cations, bile salts and the enzyme,
the pH of the buffer and the temperature are adjusted to their optima so that an appreciable degree of hydrolysis
(50-60% is sufficient) occurs in a short time.
Undesirable side reactions such as acyl migration are thereby minimized.
A semi-micro method developed by Luddy et al. [3] is recommended as the best practical procedure for the purpose. In essence, a buffer solution (pH 8) containing bile salts and calcium chloride is added to the triacylglycerols at 40°C. After a brief equilibration period, the enzyme preparation is added and the mixture is shaken very vigorously for 1-2 minutes, when about 50% hydrolysis should have occurred. The reaction is then stopped by addition of acid, the lipid products are extracted, and the monoacylglycerols are isolated by thin-layer chromatography for methylation and GC analysis of their fatty acid components.
As hydrolysis may not be completely random and as there may be some contamination from lipids endogenous to the enzyme preparation, or from fatty acids liberated from position sn-2 following acyl migration, the free fatty acids released may be somewhat different from the composition originally present in the primary positions of the triacylglycerols. The mean composition of each fatty acid in positions sn-1 and sn-3 must be calculated from its concentrations in the intact triacylglycerol and in position sn-2 by means of the relationship -
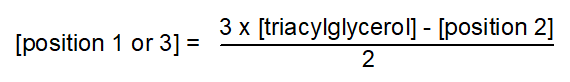
The mould Rhizopus arrhizus secretes an extracellular lipase, which has an absolute specificity for the primary bonds of glycerolipids and so resembles pancreatic lipase in many respects. It differs in that it does not have an absolute requirement for calcium ions and is not activated by bile salts. Although it offers no appreciable advantages over pancreatic lipase in most instances, there are a few applications to triacylglycerols where it has been preferred, and it can be used for positional distributions in complex polar glycerolipids. Some other enzymes from fungal sources have some limited regio/stereo bias.
3.2. Regiospecific Analysis of Triacyl-sn-glycerols -
Chemical and Spectroscopic Means
A chemical procedure for regiospecific analysis triacylglycerols has been described that utilizes partial deacylation with ethyl magnesium bromide (see next section) followed by derivatization with n-butyryl chloride. The dibutyrate derivatives of monoacylglycerols are then analysed directly by GC on a polar stationary phase (65% phenylmethylsilicone), which permits resolution of the 1(3)- from the 2‑monoacylglycerol derivatives [4]. The procedure has been used for triacylglycerols with a range of fatty acid components, i.e., C12 to C20 in chain length, and with double bonds close to the carboxyl group where pancreatic lipase hydrolysis is less suitable.
13C-Nuclear magnetic resonance (NMR) spectroscopy can be used to obtain quantitative information on the distribution of fatty acids in position 2 of triacyl-sn-glycerols, because of small shifts associated with the first few carbons of the fatty acids, depending on whether they are on positions 1/3 or 2. It appears to be most useful for fish oils, in which fatty acids have double bonds close to the carboxyl group and are not easily analysed by other means. The procedure is non-destructive, but among some disadvantages, it does not distinguish between different saturated components, or sometimes between unsaturated fatty acids with the first double bond in the same position [5,6].
Modern mass spectrometry methods, especially with electrospray and other soft ionization techniques, can be used to determine the regiospecific distribution of fatty acids within triacylglycerols, but further discussion would require a much longer and more detailed web page. In combination with HPLC, this is a powerful approach to the determination of triacylglycerol structures (for a review, see [7] - alas, I retired too early to have personal experience of this methodology!). Stereospecific analysis is not possible by this means.
3.3. Stereospecific Analysis of Triacyl-sn-glycerols -
Methods using Stereospecific Enzymes
While no lipase has yet been isolated that is capable of distinguishing between position 1 and 3 of a triacyl-sn-glycerol, ingenious stereospecific analysis procedures have been developed for determining the compositions of each of positions sn-1, sn-2 and sn-3. Several methods have been described over the years, but nowadays there are two main approaches that make use of stereospecific enzymes after hydrolytic/synthetic steps.
Hans Brockerhoff [8] devised the first stereospecific analysis procedure of general applicability, and although it has been much modified by subsequent research workers, in essence it has stood the test of time. The adaptation [9] is generally recommended as it affords other analytical opportunities, and the basic procedure is outlined below. α,β‑Diacylglycerols (an equimolar mixture of the 1,2- and 2,3-sn-isomers) are first prepared by partial hydrolysis for conversion synthetically to phospholipid derivatives, which are in turn hydrolysed by the phospholipase A2 of snake venom, an enzyme which reacts only with the "natural" 1,2-diacyl-sn-glycerophosphatide. The products are a lysophosphatide that contains the fatty acids originally present in position sn-1, free fatty acids released from position sn-2, and the unchanged "unnatural" 2,3-diacyl-sn-phosphatide. After isolation of each product by TLC and transesterification, the fatty acid compositions (in mol per cent) are determined by GC. The composition of position sn-2 can be determined independently by means of pancreatic lipolysis as a check.
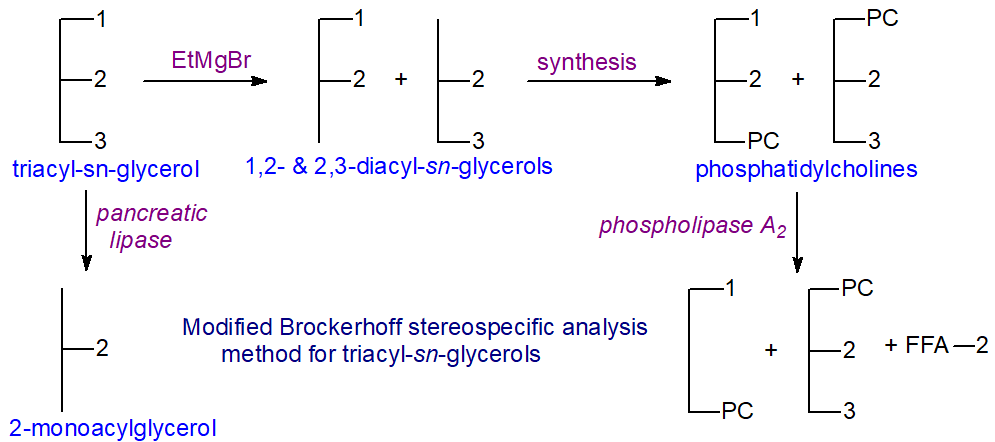
Only the fatty acid composition of position sn-3 is not determined directly by this method, but the amount of each fatty acid in this position can be calculated from the analysis of the original triacylglycerol and those of positions sn-1 and sn-2, or from the analysis of the 2,3‑diacyl-sn-phosphatide, i.e., for each fatty acid component -

The key to success with the procedure lies in the preparation of the intermediate α,β-diacylglycerols, which must be generated in a random manner so that the fatty acid compositions of the various positions are identical to those in the original triacylglycerols. There must be no selectivity for specific fatty acids or fatty acid combinations and the least possible acyl migration during their formation. Hydrolysis with pancreatic lipase was used initially but a Grignard reagent, ethyl magnesium bromide, is now preferred as it has no known fatty acid specificities and causes less acyl migration than other methods tested. In practice, such acyl migration as does occur causes some contamination (6-10%) of the 1,3-diacylglycerols but much less of the α,β‑diacylglycerols in which the primary positions are virtually unchanged [10]. I must acknowledge that I am not convinced that the problem has been fully solved, especially with fish oils where double bonds are close to the carboxyl group. The α,β-diacylglycerols required for the procedure must not come in contact with polar solvents or be heated, and they must be isolated immediately by means of TLC on layers of silica gel G impregnated with boric acid for conversion without delay to phosphatides, which can in turn be purified by TLC or other methods.
In the original Brockerhoff procedure, phosphatidylphenols were prepared from the diacylglycerols, but it was later shown that it is relatively easy to prepare phosphatidylcholines, the chromatographic properties of which are better understood. These can also be hydrolysed with some stereo-selectivity with phospholipase C, offering a further analytical option.
Although the procedure is rather complex and has several steps, it is capable of reasonable precision in the hands of skilled workers. Analyses should not be accepted unless the results for major components in positions sn-2 and sn-3, determined by both available methods, agree within 4% (absolute). The main drawback to this approach to the stereospecific analysis of triacyl-sn-glycerols is that the fatty acid composition of position sn-3 is not determined directly, so that the proportionate errors in the results for minor fatty acids in this position can be considerable. Small negative values are sometimes obtained, and small positive values may arise in calculations for a component that is not present in the position. This could no doubt be overcome by reacting the unchanged 2,3‑diacyl-sn-phosphatide with the lipase of Rhizopus arrhizus to release the fatty acids from position sn-3 for direct analysis.
An alternative procedure that uses a stereospecific enzyme has been described that looks interesting, although it does not yet appear to have been used outwith the laboratory of the originators [11]. A mixture of 1,2- and 2,3-diacyl-sn-glycerols (DAG) are prepared by reaction with a Grignard reagent, and these are incubated with the sn-1,2-diacylglycerol kinase from Escherichia coli (available commercially) and ATP. The 1,2-DAG are converted to 1,2-diacyl-sn-glycerol phosphate (phosphatidic acid), while the 2,3-DAG remain unchanged. The products are isolated by TLC for the determination of their fatty acid compositions, and that for position sn-2 is obtained by a separate analysis of the triacylglycerols by the pancreatic lipase procedure. Calculation of the compositions of the three positions is then a matter of simple arithmetic.

3.4. Stereospecific Analysis of Triacyl-sn-glycerols -
Methods employing Chiral Chromatography
Very different approaches to stereospecific analysis of triacyl-sn-glycerols have been described that use simple chemical degradative and derivatization steps and the methodology of chiral chromatography. One such method was developed in our laboratory. The principle is dependent on the fact that diastereomeric compounds can be resolved by adsorption chromatography on silica gel because they have different physical and chemical properties. Thus a chiral derivatizing agent (R)-X will react with a racemic substance (R,S)-Y to form diastereomeric products as follows.
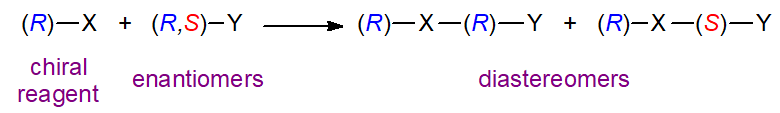
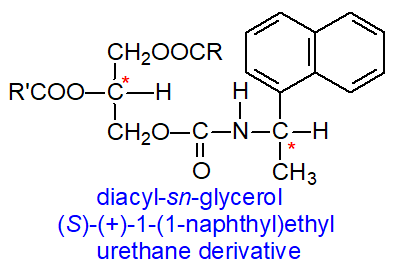
The degree of separation of the two diastereomers in a chromatographic system will depend on the chiral structures of X and Y and the manner of their interactions with the mobile and stationary phases. It is noteworthy that the order of elution of the diastereomeric derivatives is reversed if the other enantiomer of the reagent, i.e., (S)-X, can be employed. (S)-(+)-1-(1-Naphthyl)ethyl urethane derivatives of diacyl-sn-glycerols are preferred for the purpose. With the HPLC column of silica gel used in this work, it appears that the presence of the hydrogen atom on the nitrogen between the chiral centres is essential to the separation process, and it is presumed to be a primary site for hydrogen bonding to silanols on the adsorbent surface.
 The first step in the stereospecific analysis procedure
is identical to that in most other methods, i.e., partial hydrolysis of the triglycerides with ethyl magnesium bromide to a mixture of
sn-1,2-, 2,3- and 1,3-diacylglycerols, amongst other products [12,13].
Next, the products are reacted with a chiral derivatizing agent, (S)-(+)-1-(1-naphthyl)ethyl isocyanate
and the diacyl-sn-glycerol urethane derivatives are isolated by chromatography on solid-phase
extraction columns containing an octadecylsilyl phase; by-products of low molecular-weight and excess derivatizing reagents
are eluted first and discarded, before the required diacylglycerol urethane derivatives are recovered.
The first step in the stereospecific analysis procedure
is identical to that in most other methods, i.e., partial hydrolysis of the triglycerides with ethyl magnesium bromide to a mixture of
sn-1,2-, 2,3- and 1,3-diacylglycerols, amongst other products [12,13].
Next, the products are reacted with a chiral derivatizing agent, (S)-(+)-1-(1-naphthyl)ethyl isocyanate
and the diacyl-sn-glycerol urethane derivatives are isolated by chromatography on solid-phase
extraction columns containing an octadecylsilyl phase; by-products of low molecular-weight and excess derivatizing reagents
are eluted first and discarded, before the required diacylglycerol urethane derivatives are recovered.
The most important step involves resolution of the diacylglycerol urethanes by HPLC on columns of silica gel. A simple isocratic mobile phase is employed, and as the derivatives absorbed strongly in the UV spectrum, detection is straightforward. 1,3‑Diacyl-sn-glycerol (1,3-DAG) urethanes elute early, and this fraction is easily recovered, but as they are more susceptible to acyl migration than the other products of interest, they are not used further. As the derivatizing agent is chiral and a single enantiomer, the 1,2- and 2,3‑diacyl-sn-glycerol urethanes formed from it are now diastereomers, so they are separable in a non-chiral environment.
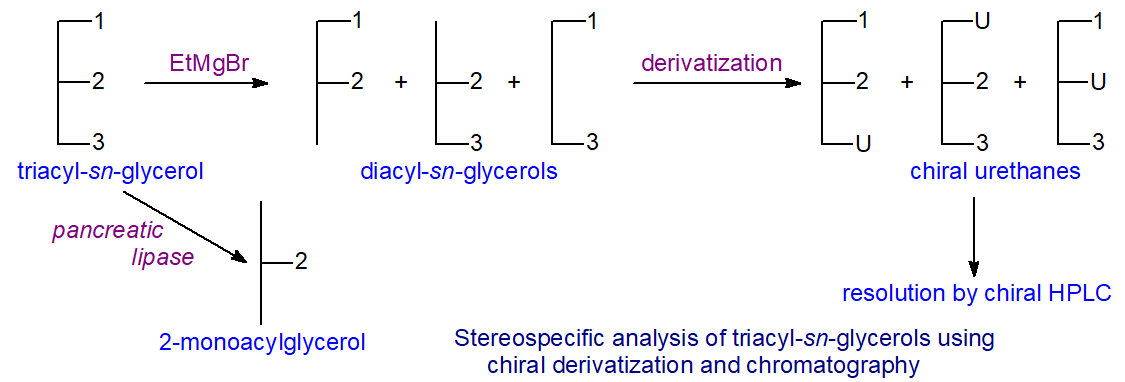
In practice, the 1,2-diacyl-sn-glycerol (1,2-DAG) derivatives elute ahead of the 2,3-diastereomers (2,3-DAG), and the two distinct fractions can be collected. Some separation of molecular species can occur within each diastereomeric fraction, and while this might be considered an advantage in some circumstances, e.g., for mass spectrometric analysis, it can be something of a nuisance as it restricts the range of fatty acid components in the triacylglycerols that can be investigated by spoiling the resolution between fractions. Fortunately, most of the common fats with C16 and C18 fatty acids are in the practical range, and it may be possible with further development to extend this eventually. The separation by chiral HPLC of diacyl-sn-glycerol urethane derivatives prepared from palm oil is illustrated.
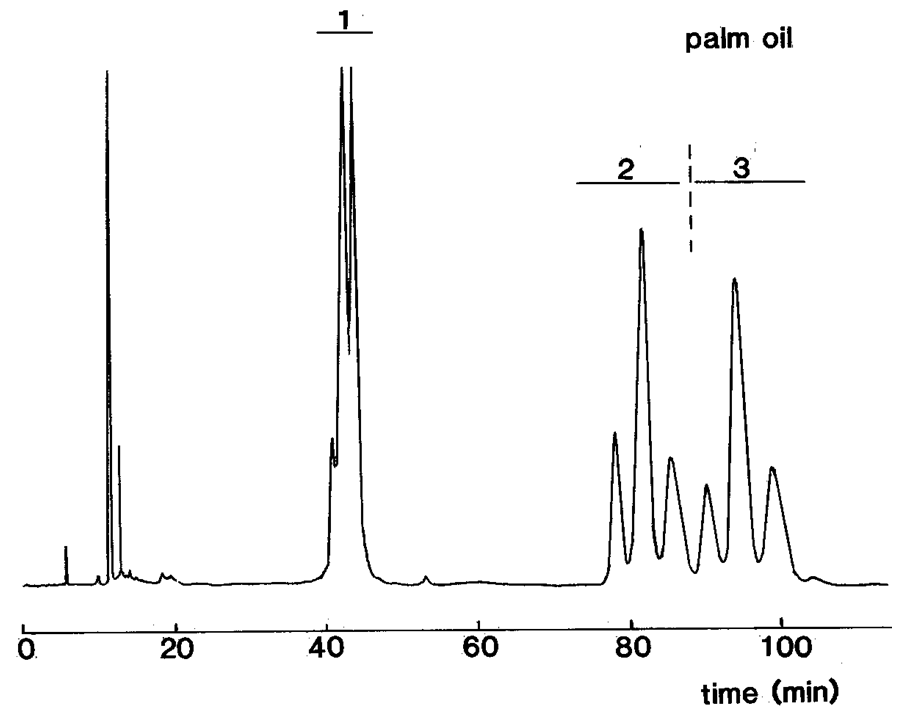 |
Separation of (S)-(+)-1-(1-naphthyl)ethyl urethane derivatives of diacyl-sn-glycerols (DAG) from palm oil by HPLC on two columns of silica gel in series (HypersilTM 3μ, 250 x 4.6 mm) with isooctane with 0.3% isopropanol (containing 2% water) as mobile phase [12] (Abbreviations: 1 = 1,3-DAG, 2 = 1,2-DAG, 3 = 2,3-DAG. Reproduced by kind permission of the Journal of the American Oil Chemists’ Society). |
The final step requires methylation of each of the fractions for analysis by GC with the highest precision possible, after which the results for the positional distributions are simply a matter for calculation. As the fatty acid composition of the intact triacylglycerols is known, and that of the 1,2‑diacyl-sn-glycerol derivatives has been determined, it is straightforward arithmetic to calculate the composition of position sn-3. Similarly, the composition of position sn-1 can be calculated once that of the 2,3-diacyl-sn-glycerols is known. That of position sn-2 is calculated by difference (or by an independent hydrolysis with pancreatic lipase). Thus, the composition of all three positions can be determined without resort to enzymes by using standard chromatography columns and derivatizing agents that are available from commercial sources.
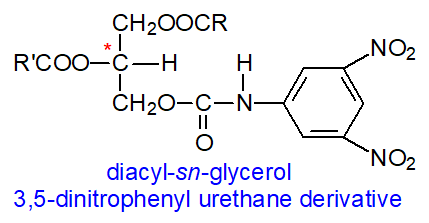 Professor Toru Takagi and colleagues in Japan adopted a related procedure in which
they converted mono- and diacyl-sn-glycerols prepared from triacylglycerols to the 3,5‑dinitrophenyl urethane
(DNPU) derivatives for resolution by HPLC on columns containing a stationary phase with chiral moieties bonded
chemically to a base of silica gel (e.g., SumipaxTM OA-4100).
The 3,5-dinitrophenyl moieties of the urethanes contribute to charge-transfer interactions with functional groups having
pi electrons on the stationary phase and thus aid resolution.
Professor Toru Takagi and colleagues in Japan adopted a related procedure in which
they converted mono- and diacyl-sn-glycerols prepared from triacylglycerols to the 3,5‑dinitrophenyl urethane
(DNPU) derivatives for resolution by HPLC on columns containing a stationary phase with chiral moieties bonded
chemically to a base of silica gel (e.g., SumipaxTM OA-4100).
The 3,5-dinitrophenyl moieties of the urethanes contribute to charge-transfer interactions with functional groups having
pi electrons on the stationary phase and thus aid resolution.
Although diacylglycerols could be used in a manner analogous to the above, in their preferred procedure for stereospecific analysis [14-16], 1-, 2- and 3-monoacyl-sn-glycerols are prepared from triacyl-sn-glycerols by partial hydrolysis with ethyl magnesium bromide. The 1(3)-forms are separated from the 2-isomers by TLC on silica gel impregnated with boric acid, before being converted to the DNPU derivatives. Then, the 1- and 3-monoacyl-sn-glycerol derivatives are resolved on a chiral HPLC column. The distributions of fatty acids in each of positions sn-1, sn-2 and sn-3 are obtained following methylation and GC analysis. By means of lowering the column temperature and slowing down the flowrate, the method can even be applied to such complex triacyl-sn-glycerols as fish oils.
This last method has the virtue of enabling direct determination of the composition of all three positions, but I would be concerned that the monoacylglycerols were generated from triacylglycerols by the action of the Grignard reagent in a truly random manner, although this is claimed to be the case.
Some separations of intact enantiomeric triacyl-sn-glycerols have now been achieved by chiral phase chromatography with recent emphasis on polysaccharide-based chiral stationary phases incorporating either amylose- or cellulose-phenylcarbamate derivatives as chiral selectors [17-20]. Such methods may still fall short of a complete stereospecific analysis of natural fats, but they hold promise for the future.
3.5. Molecular Species Composition of Triacylglycerols
It is well known that natural triacylglycerols exist in the form of innumerable molecular species, and for example, a triacylglycerol with only five different fatty acid constituents may consist of 75 different molecular species not including enantiomers, or 125 if enantiomers are included. With milk fat, the number of different molecular species reaches astronomical proportions. Mass spectrometric methods are now favoured for analysis with the possibility for regiospecific analysis, and these can be combined with HPLC for increased resolution, either in the form of silver ion or reversed-phase chromatography [21]. This is too big a topic for further discussion here.
One approach to combining stereospecific data with those on molecular species has been to subject molecular fractions obtained by silver ion chromatography to stereospecific analysis, and this has given some interesting results for olive oil [22]. Others have used a combination of reversed-phase and chiral-phase chromatography in combination with mass spectrometry with impressive results [23].
Other web pages on this site dealing with triacylglycerols are Triacylglycerols: Part 1 - their structure and compositions and Triacylglycerols: Part 2 - their biosynthesis and metabolism.
References
- Kuksis, A. and Itabashi, Y. Regio- and stereospecific analysis of glycerolipids. Methods, 36, 172-185 (2005); DOI.
- Kuksis, A. and Itabashi, Y. LC/MS and chiral separation. In: Lipid Analysis and Lipidomics: New Techniques and Applications, pp. 73-108 (Ed: M.M. Mossoba, J.K.G. Kramer, J.T. Brenna and R.E. McDonald, AOCS Press, Champaign, USA). (2006).
- Luddy, F.E., Barford, R.A., Herb, S.F., Magidman, P. and Riemenschneider, R.W. Pancreatic lipase hydrolysis of triglycerides by a semimicro technique. J. Am. Oil Chem. Soc., 41, 693-696 (1964); DOI.
- Angers, P. and Arul, O. A simple method for regiospecific analysis of triacylglycerols by gas chromatography. J. Am. Oil Chem. Soc., 76, 481-484 (1999); DOI.
- Gunstone, F.D. High resolution 13C-NMR spectroscopy of lipids. In: Advances in Lipid Methodology - Two, pp. 1-68 (Ed. W.W. Christie, Oily Press, Dundee) (1993).
- Standal, I.B., Axelson, D.E. and Aursand, M. Differentiation of fish oils according to species by 13C-NMR regiospecific analyses of triacylglycerols. J. Am. Oil Chem. Soc., 86, 401-407 (2009); DOI.
- Fabritius, M. and Yang, B.R. Analysis of triacylglycerol and phospholipid sn-positional isomers by liquid chromatographic and mass spectrometric methodologies. Mass Spectrom. Rev., 1-33 (2023); DOI.
- Brockerhoff, H. A stereospecific analysis of triglycerides. J. Lipid Res., 6, 10-15 (1965); DOI.
- Myher, J.J. and Kuksis, A. Stereospecific analysis of triacylglycerols via racemic phosphatidylcholines and phospholipase C. Can. J. Biochem., 57, 117-124 (1979); DOI.
- Christie, W.W. and Moore, J.H. A semimicro method for the stereospecific analysis of triglycerides. Biochim. Biophys. Acta, Lipids, 176, 445-452 (1969); DOI.
- Simonetti, M.S., Damiani, F., Gabrielli, L., Cossignani, L., Blasi, F., Marini, F., Montesano, D., Maurizi, A., Ventura, E., Bosi, A. and Damiani, P. Characterization of triacylglycerols in Arbutus unedo L. seeds. Ital. J. Food Sci., 20, 49-56 (2008).
- Laakso, P. and Christie, W.W. Chromatographic resolution of chiral diacylglycerol derivatives: potential in the stereospecific analysis of triacyl-sn-glycerols. Lipids, 25, 349-353 (1990); DOI.
- Christie, W.W., Nikolova-Damyanova, B., Laakso, P. and Herslof, B. Stereospecific analysis of triacyl-sn-glycerols via resolution of diastereomeric diacylglycerol derivatives by high-performance liquid chromatography on silica. J. Am. Oil Chem. Soc., 68, 695-701 (1991); DOI.
- Takagi, T. and Ando, Y. Stereospecific analysis of acyl group distribution in triacylglycerols by HPLC with chiral column. J. Japan Oil Chem. Soc. (Yukagaku), 39, 622-628 (1990); DOI.
- Takagi, T. and Ando, Y. Stereospecific analysis of triacyl-sn-glycerols by chiral HPLC. Lipids, 26, 542-547 (1991); DOI.
- Takagi, T. and Ando, Y. Stereospecific analysis of triacylglycerols by chiral-phase HPLC. Direct derivatization of partially hydrolyzed products. J. Japan Oil Chem. Soc. (Yukagaku), 40, 288-292 (1991); DOI.
- Lísa, M. and Holčapek, M. Characterization of triacylglycerol enantiomers using chiral HPLC/APCI-MS and synthesis of enantiomeric triacylglycerols. Anal. Chem., 85, 1852-1859 (2013); DOI.
- Nagai, T., Kinoshita, T., Kasamatsu, E., Yoshinaga, K., Mizobe, H., Yoshida, A., Itabashi, Y. and Gotoh, N. Simultaneous quantification of mixed-acid triacylglycerol positional isomers and enantiomers in palm oil and lard by chiral high-performance liquid chromatography coupled with mass spectrometry. Symmetry-Basel, 12, 1385 (2020); DOI.
- Řezanka, T., Lukavský, J., Rozmoš, M., Nedbalová, L. and Jansa, J. Separation of triacylglycerols containing positional isomers of hexadecenoic acids by enantiomeric liquid chromatography-mass spectrometry. J. Chromatogr. B, 1208, 123401 (2022); DOI.
- Ianni, F., Carotti, A., Protti, M., Favilli, A., Gerli, S., Furlanetto, S., Mercolini, L. and Sardella, R. Chiral high-performance liquid chromatography analysis of mono-, di-, and triacylglycerols with amylose- and cellulose-phenylcarbamate-based stationary phases. J. Pharm. Biomed. Anal., 236, 115720 (2023); DOI.
- Christie, W.W. and Han, X. Lipid Analysis - Isolation, Separation, Identification and Lipidomic Analysis (4th edition), 446 pages (Oily Press, Woodhead Publishing and now Elsevier) (2010) - see Science Direct.
- Santinelli, F., Damiani, P. and Christie, W.W. The triacylglycerol structure of olive oil determined by silver ion high-performance liquid chromatography in combination with stereospecific analysis. J. Am. Oil Chem. Soc., 69, 552-556 (1992); DOI.
- Kalpio, M., Linderborg, K.M., Fabritius, M., Kallio, H. and Yang, B.R. Strategy for stereospecific characterization of natural triacylglycerols using multidimensional chromatography and mass spectrometry. J. Chromatogr. A, 1641, 461992 (2021); DOI.
Also of interest -
- Park, J.Y. and Park, K.M. Lipase and its unique selectivity: a mini-review. J. Chem., 7609019 (2022); DOI.
 |
© Author: William W. Christie |  |
|
| Contact/credits/disclaimer | Updated: May 8th, 2024 | ||
